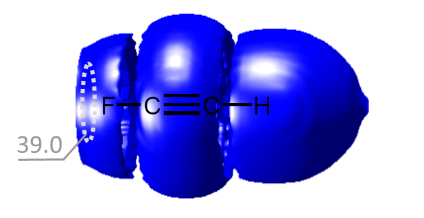Научная группа профессора П.М. Толстого
Научная группа кафедры физической органической химии
Лаборатория невалентных взаимодействий
Состав группы
 |
Руководитель группы Пётр Михайлович Толстойд.х.н., профессор http://www.researcherid.com/rid/J-2966-2013 peter.tolstoy@spbu.ru |
|
 |
Елена Юрьевна Тупикинак.ф.-м.н., доцент https://orcid.org/0000-0002-0998-8348 e.tupikina@spbu.ru |
|
 |
Валерия Вячеславовна Муллояровак.х.н., ассистент http://orcid.org/0000-0002-5688-2431 v.mulloyarova@spbu.ru |
|
|
||
 |
Цыбулин Семён Валерьевичк.х.н., научный сотрудник https://orcid.org/0000-0001-5033-8454 пом. 2135, 2141 |
 |
Омар Алкхудераспирант, инженер-исследователь пом. 2135, 2130 |
 |
Валерий Александрович Верховаспирант, инженер-исследователь пом.2135, 2126, 2142 |
 |
Губанова Александра Николаевнастудент бакалавриата пом. 2135, 2142 |
 |
Антон Сергеевич Захаровстудент бакалавриата пом. 2135, 2130 |
 |
Камынин Денис Анатольевичстудент бакалавриата пом.2135, 2141 |
 |
Марк Валерьевич Капланскийстудент магистратуры, инженер-исследователь пом. 2126
|
 |
Данил Вячеславович Крутинаспирант, инженер-исследователь пом. 2135, 2126 |
 |
Степан Алексеевич Мешалкинстудент бакалавриата, лаборант-исследователь пом. 2135, 2126, 2141 |
 |
Морозова Ксения Игоревнастудент магистратуры st117583@student.spbu.ru пом.2135, 2130 |
 |
Анна Александровна Титовастудент магистратуры, инженер-исследователь |
 |
Эдем Рустемович Чакаловаспирант, инженер-исследователь |
 |
Даниил Алексеевич Шитовстудент магистратуры пом. 2126 |
Дмитрий Сергеевич Лобановстудент магистратуры st133229@student.spbu.ru пом. 2135, 2142 |
|
Александра Николаевна Губановастудент бакалавриата пом. 2135, 2142 |
|
Максим Леонидович Кругловстудент бакалавриата st106286@student.spbu.ru пом. 2135, 2142 |
|
Степан Андреевич Галиахметовстудент бакалавриата st117534@student.spbu.ru пом.2135, 2142 |
|
Антон Павлович Годунстудент бакалавриата st116496@student.spbu.ru пом. 2135 |
Бывшие студенты и сотрудники
 |
Александр Сергеевич Антоновк.х.н., исследователь University of Regensburg https://orcid.org/0000-0001-7047-789X |
 |
Виктор Геннадьевич БардаковВыпускник программы аспирантуры |
 |
Иван Сергеевич Гибавыпускник аспирантуры к.ф.-м.н. |
 |
Александра Александровна Ефимовамагистр физики |
 |
Михаил Андреевич Золенко |
 |
Валерий Владимирович Карповмагистр химии |
 |
Владислав Олегович Коростелёвмагистр химии |
 |
Владимир Юрьевич МикшиевК.Х.Н. https://orcid.org/0000-0003-1204-0352 |
 |
Александра Михайловна Пузы́кмагистр химии |
 |
Денис Владимирович Соловьёв |
 |
Андрей Алексеевич Сударкин |
 |
Илья Александрович Татаринов |
 |
Дмитрий Олегович Толоченкомагистр химии |
 |
Дарья Олеговна Устимчукбакалавр химии |
 |
Иван Сергеевич Чунарев |
 |
Йоханнес ЭдерМагистрхимии(Regensburg university) |
 |
Артём Алексеевич Якубенкобакалавр химии |
 |
Алексей Сергеевич Острасьбакалавр химии |
 |
Анастасия Владимировна Наммагистр химии |
Сотрудничество
| Совместные публикации | |
|
Александр Антонов, к.х.н., исследователь, Университет Регенсбурга, Германия |
A.S. Zakharov, D.V. Krutin, P.O. Mosalyov, E.Yu. Tupikina, A.S. Antonov, P.M. Tolstoy, V.V. Mulloyarova, “Phosphine Selenides: Versatile NMR Probes for Analyzing Hydrogen OH···Se and Halogen I···Se Bonds”, Phys.Chem.Chem.Phys.2024, published online. DOI: 10.1039/D4CP01895H. J. Eder, A.S. Antonov, E.Yu. Tupikina, R.M. Gschwind, “Chiral Diselenophosphoric Acids for Ion Pair Catalysis: A Novel Approach to Enhance Both Proton Donating and Proton Accepting Properties”, Chem. Eur. J. 2024, e202401793. DOI: 10.1002/chem.20240179. S.A. Meshalkin, S.V. Tsybulin, V.G. Bardakov, I.A. Tatarinov, D.A. Shitov, E.Yu. Tupikina, M.M. Efremova, A.S. Antonov, “Buttressing Effect" in the Halogen-Lithium Exchange in ortho-bromo-N,N-dimethylanilines and Related Naphthalenes”, Chem. Eur. J. 2023, e202303956. DOI: 10.1002/chem.202303956. M.V. Kaplanskiy, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “NMR Descriptors of the Strained Metallacycles Formation in Organo-lithiums: Theoretical Study”, Organic and Biomolecular Chemistry2023. DOI: 10.1039/d3ob01916k. A. A. Yakubenko, E. Yu. Tupikina, A. S. Antonov, “The Role of Conjugation in the Halogen‐Lithium Exchange Selectivity: lithiation of 4, 6, 7, 9‐tetrabromo‐1, 3‐dimethyl‐2, 3‐dihydro‐1H‐perimidine”, Chem. Eur. J. 2023, 29, e202301439. DOI: 10.1002/chem.202301439. |
|
Вадим Юрьевич Кукушкин проф., акад. РАН, д.х.н. Санкт-Петербургский государственный университет Химический институт Кафедра физической и органической химии |
A.VRozhkov, E.Yu. Tupikina, K.I. Tugashov, V.Yu. Kukushkin, « PureHeterometallicSpodiumBonding», CrystEngComm2024. DOI: 10.1039/D4CE00825A. M.V. Kashina, M. A. Kinzhalov, E.Yu. Tupikina, V.Yu. Kukushkin, “Linear and Bent Noncovalent M··· S═ C═ N–M′ Interactions: The Case of Palladium (II) and Platinum (II) Thiocyanate Species”, Cryst. Growth Des.2023, 23, 4322–4335. DOI: 10.1021/acs.cgd.3c00116. I.S. Aliyarova, E.Yu. Tupikina, N.S. Soldatova, D.M. Ivanov, P.S. Postnikov, M. Yusubov, V. Yu. Kukushkin, «Halogen Bonding Involving Gold Nucleophiles in Different Oxidation States», Inorg. Chem.2022, 61, 39, 15398–15407; DOI: 10.1021/acs.inorgchem.2c01858. |
|
АлександрФёдоровичПожарский проф., д.х.н. Южный Федеральный Университет |
A.V. Marchenko, V.A. Ozeryanskii, O.P. Demidov, A.A. Antonov, E.Yu. Tupikina, A.F. Pozharskii, “Organometallic Synthesis of 2,3,6,7-Tetrasubstituted 1,8-Bis(dimethylamino)naphthalenes for Investigation of the Double Buttressing Effect in Proton Sponges”, J. Org. Chem.2022, 87(24):16506-16516. DOI: 10.1021/acs.joc.2c02212 |
|
Марк Сигалов проф., д.х.н. Университет Бен-Гуриона, Израиль |
E.Yu. Tupikina, M.V. Sigalov, O. Alkhuder, P.M. Tolstoy, “Charge Relay Without Proton Transfer: Coupling of Two Short Hydrogen Bonds via Imidazole in Models of Catalytic Triad of Serine Protease Active Site”, ChemPhysChem2024. DOI: 10.1002/cphc.202300970 E.Yu. Tupikina, M.V. Sigalov, P.M. Tolstoy, «Simultaneous Estimation of Two Coupled Hydrogen Bond Geometries from Pairs of Entangled NMR Parameters: The Test Case of 4-Hydroxypyridine Anion», Molecules2022, 27, 3923. DOI: 10.3390/molecules27123923. |
|
Костас Сотриадис проф. Институт теоретической и прикладной механики Чешской академии наук, Прага, Чешская Республика |
K. Sotiriadis, A.S. Mazur, Peter M. Tolstoy, P. Mácová, A. Viani, “External magnesium sulfate attack on hydrated Portland-limestone cements: Effect of the concurrent presence of sodium chloride in the corrosive environment and metakaolin admixture in the binder”, Cem. Concr. Compos.2024, 151, 105614. DOI: 10.1016/j.cemconcomp.2024.105614. A.Mazur, P. Tolstoy, K. Sotiriadis, “13С, 27Al and 29Si NMR investigation of the hydration kinetics of Portland-limestone cement pastes containing CH3-COO–R+ (R=H or Na)”, Materials 2022, 15, 2004. DOI: 10.3390/ma15062004. K. Sotiriadis, K. Aspiotis, A. Mazur, P. Tolstoy, E. Badogiannis, S. Tsivilis, “Characterization of Old Concrete from a Heritage Structure of Inousses Cluster of Islands”, Lect. Notes Civ. Eng.2022, 209, 80-89. DOI: 10.1007/978-3-030-90788-4_7. |
|
Виктор Розенцвет проф., д.х.н. Институт экологии бассейна реки Волга, Российская академия наук, Тольятти, Россия |
V.A. Rozentsvet, N.A. Sablina,D.M. Ulyanova, P.M. Tolstoy, “Structural characterization of lowmolecular weight polybutadiene synthesized using cationic initiationsystem”, Russ. Chem. Bull. 2024, 73(4), 1035-1045. DOI: 10.1007/s11172-024-4218-6. V.A. Rozentsvet, D.M. Ulyanova, N.A. Sablina, S.V. Kostjuk, P.M. Tolstoy, I.A. Novakov, “Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes”, Polym. Chem.2022, 3, 1596-1607. DOI: 10.1039/d1py01684a. V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull.2022, 79, 1239-1256. DOI: 10.1007/s00289-021-03549-5. |
|
Александр Артемьев проф., д.х.н. Институт неорганической химии им. А.Н. Николаева Сибирского отделения Российской академии наук, Новосибирск, Россия |
E.Yu. Tupikina, M.P. Davydova, V.V. Mulloyarova, T.S. Sukhikh, D.G. Samsonenko, P.M. Tolstoy, A.V. Artem’ev, “Remarkably short intermolecular Se···Se contacts in Ni(II)diselenophosphinates: interplay of electrostatic and dispersion”, Inorg. Chem. Front.2024, under review |
|
Елена Валерьевна Грачёва проф., д.х.н. Санкт-Петербургский государственный университет Химический институт Кафедра общей и неорганической химии
|
A. Paderina, S. Slavova, E. Tupikina, D. Snetkov, E. Grachova, «Aggregation game: changing solid-state emission using different counterions in mono-alkynylphosphoniumPt(II) complexes », Inorg. Chem.2024. DOI: 10.1021/acs.inorgchem.4c02130. |
Направления научных исследований
Невалентные взаимодействия — это общее название взаимодействий при образовании которых не происходит обобществления электронной плотности. Энергия невалентных взаимодействий значительно меньше энергии валентных взаимодействий. Однако ввиду своей распространенности, невалентные взаимодействия играют фундаментальную роль в синтезе, катализе, инженерии кристаллов, электрохимии, молекулярном распознавании, биологии, материаловедении и т. д. Например, многие каталитические процессы могут проходить через переходные состояния и промежуточные стадии, стабилизируемые невалентными взаимодействиеми, которые определяют селективность и дальнейшее направление реакций. Кроме того, зачастую реакции, катализируемые комплексами металлов происходят за счет невалентных взаимодействий между исходным материалом и катализатором. Таким образом, понимание природы этих слабых взаимодействий может оказаться важным этапом в разработке новых эффективных синтетических катализаторов.
Все известные на сегодняшний день невалентные взаимодействия условно разделяют на четыре группы: электростатические, Ван-дер-Ваальсовы, гидрофобные и π-взаимодействия.
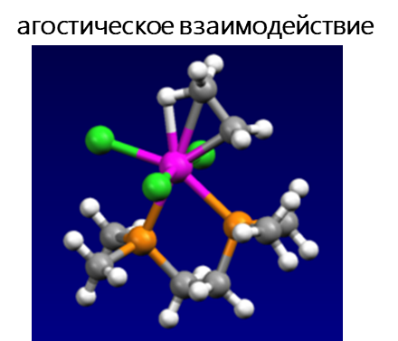
Среди электростатических взаимодействий выделяют: водородную связь, σ-дырочные (галогенные, пниктогенные, халькогенные, аэрогенные, тетрельные и пр.), агостические и ионные взаимодействия.
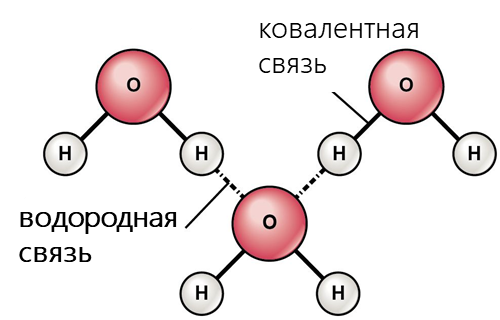
- Водородная связь возникает, когда вблизи одного электроотрицательного атома с неподеленной парой электронов оказывается атом водорода, связанный с другим электроотрицательным атомом (фтор, кислород, азот и т.д.). Эти связи обычно сильнее обычных диполь-дипольных и дисперсионных сил, но слабее, чем ковалентные и ионные связи. Водородная связь в значительной мере определяет свойства и таких биологически важных веществ, как белки и нуклеиновые кислоты. В частности, элементы вторичной структуры (например, α-спирали, β-складки) и третичной структуры в молекулах белков, РНК и ДНК стабилизированы водородными связями.
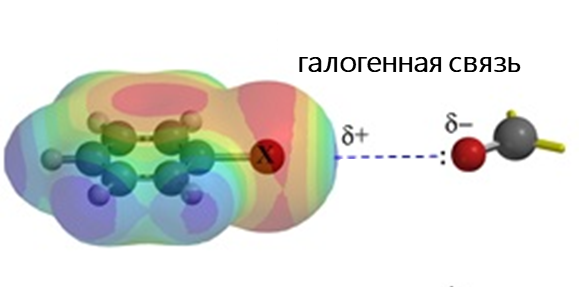
- Галогенная связь возникает за счет притягательного взаимодействия между электрофильной областью атома галогена (так называемой σ-дыркой) и нуклеофильной областью (например, неподеленной электронной парой) в другой молекуле. Это взаимодействие широко используется в таких областях, как супрамолекулярная химия, кристаллография и изготовление жидких кристаллов.
- Агостическое взаимодействие — взаимодействие координационно-ненасыщенного переходного металла с C–H связью, когда два электрона, вовлеченных в связь C–H, переходят на пустую d-орбиталь переходного металла, в результате чего образуется трехцентровая двухэлектронная связь. Многие каталитические превращения, например, окислительное присоединение и восстановительное элиминирование, предположительно протекают с участием агостических взаимодействий.
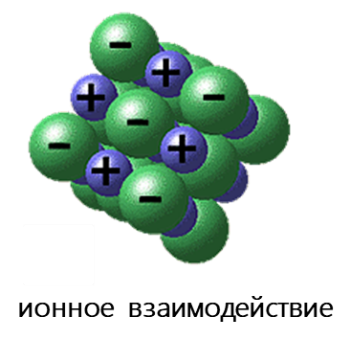
- Ионное взаимодействие реализуется за счет электростатического притяжения между анионами и катионами. Важнейшие отличия ионной связи от других типов химической связи заключаются в ненаправленности и ненасыщаемости. Именно поэтому кристаллы, образованные за счёт ионной связи, тяготеют к различным плотнейшим упаковкам соответствующих ионов.
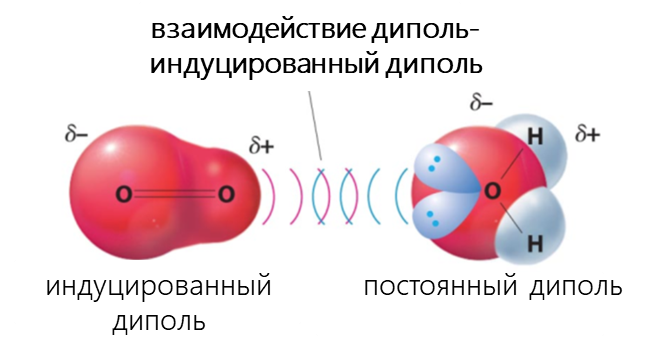
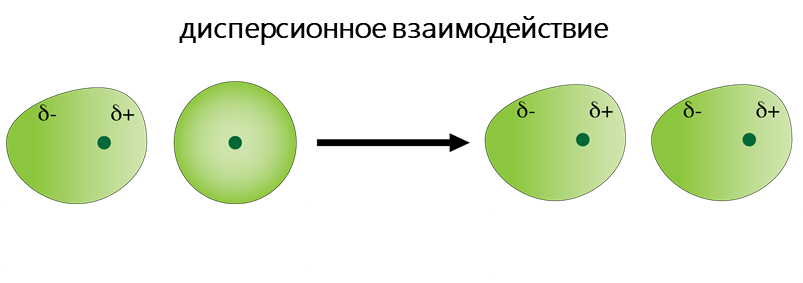
К Ван-дер-Ваальсовым относят диполь-дипольное, диполь-индуцированный диполь и дисперсионное взаимодействия.
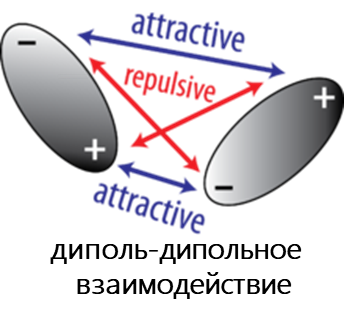
- Диполь-дипольное взаимодействие возникает, когда две полярные молекулы взаимодействуют друг с другом через пространство. При этом частично отрицательная часть одной из полярных молекул притягивается к частично положительной части второй полярной молекулы.
- Взаимодействие между диполем и индуцированным диполем возникает, когда полярная молекула индуцирует диполь в атоме или в неполярной молекуле, нарушая расположение электронов в неполярном участнике.
- Дисперсионное взаимодействие обусловлено взаимодействием атомов и молекул друг с другом за счёт их дипольных моментов, собственных или взаимоиндуцированных. Мгновенное распределение заряда одного атома или молекулы, характеризуемое мгновенным дипольным моментом, индуцирует мгновенный дипольный момент в другом атоме или молекуле
Гидрофобное взаимодействие — это притяжение между неполярными частицами в воде (или других полярных растворителях), которое обусловлено термодинамической невыгодностью контакта воды с неполярными веществами. Гидрофобное взаимодействие участвует в формировании третичной структуры белков и обеспечивает молекулярное распознавание в некоторых супрамолекулярных комплексах типа «гость–хозяин». Оно проявляется при образовании мицелл и других структур в растворах поверхностно-активных веществ.

π-взаимодействие — это тип невалентных взаимодействий, в котором богатая электронами π-система может взаимодействовать с катионом, анионом или другой π-системой. Невалентные взаимодействия с π-системами являются ключевыми для биологических процессов, таких как распознавание типа «белок-лиганд».
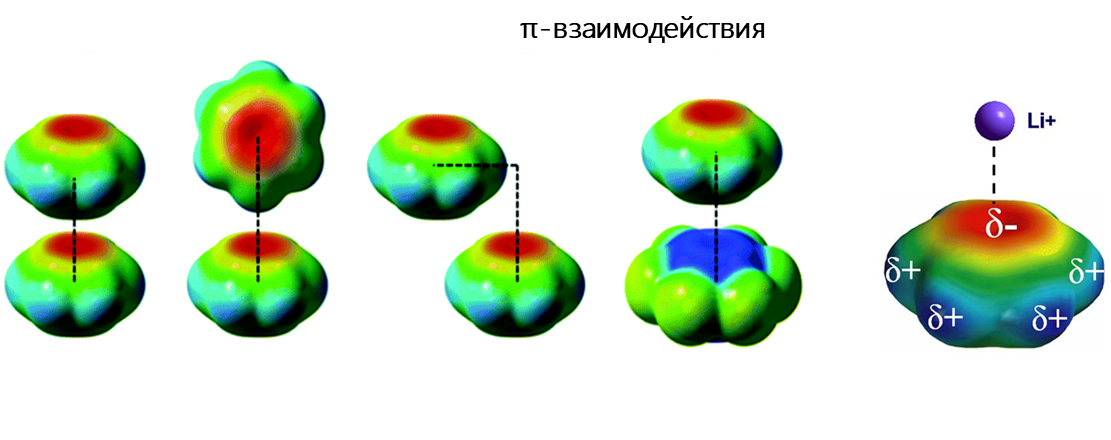
Научные проекты
Элементоорганическая химия
Со времён открытия Эдвардом Франкландом во второй половине 19 века основных принципов получения элементоорганических соединений началось бурное развитие применения этих реагентов в органическом синтезе. Сегодня элементоорганическая стратегия функционализации позволят проводить сложные превращения с высокой регио- и стереоселективностью, а также открывает возможность введения заместителей в положения недоступные методами классической химии. С целью развития синтетического потенциала элементоорганических соединений в нашей лаборатории реализуются следующие проекты:
- Прямое металлирование ароматических аминов и родственных соединений с целью синтеза их труднодоступных мета- и пери-производных.
|
Antonov, Yakubenko Synthesis 2020 |
Antonov et al. J. Organomet. Chem. 2020 |
- Исследование строения элементоорганических соединений
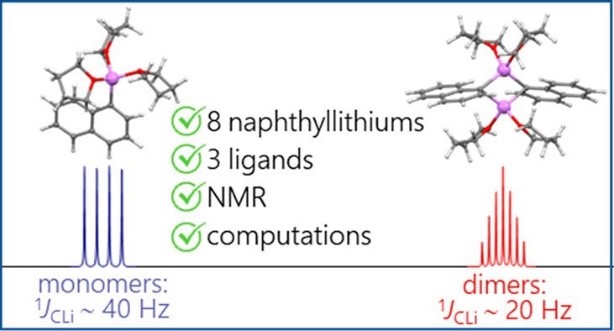
Antonov et al. Organometallics. 2020
- Стерическая активация диметиламиногруппы в синтезе полиядерных азотистых гетероциклов
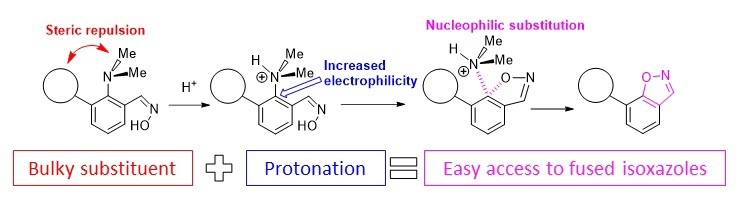
Antonov et al. Molecules 2020
Современный подход к изучению невалентных взаимодействие требует разработки методов синтеза новых доноров и акцепторов, которые позволят понять периодичность изменения силы взаимодействия от природы элементов, входящих в состав участников связывания, а также позволят использовать новые более чувствительные методы исследования. В рамках данного направления наша лаборатория реализует следующие проекты:
- Разработка методов синтеза органических кислот, оксидов и селенидов элементов 15-й группы
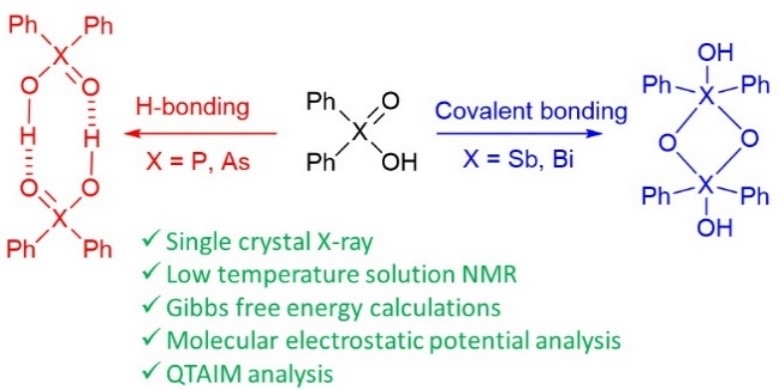
Yakubenko et al. Phys. Chem. Chem. Phys. 2022
- Получение хиральных селенофосфорных кислот и изучение их каталитической активности (совместно с Университетом Регенсбурга)
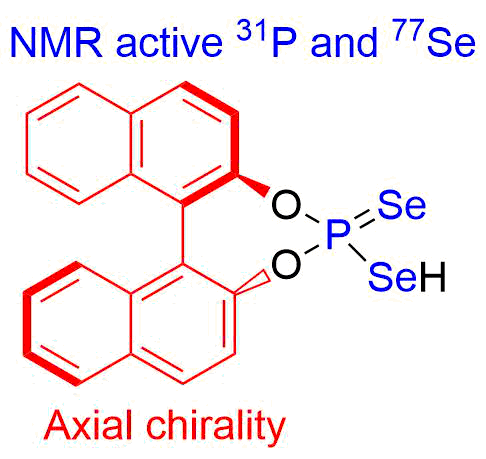
Квантовая химия
Методы квантовой химии основаны на принципах квантовой механики в применении к молекулярным системам. Решение квантово-химической задачи сводится к численному решению уравнения Шредингера с использованием различных приближений. Современные методы требуют значительных вычислительных ресурсов, поэтому расчёты проводятся с использованием мощных вычислительных кластеров.
Методы спектроскопии (ИК, ЯМР) и квантовой химии взаимно дополняют друг друга. Расчёты используются для объяснения экспериментально наблюдаемых феноменов, для предсказания результатов эксперимента или для моделирования принципиально невозможных экспериментов.Giba et al. Symmetry 2021
В нашей лаборатории мы решаем задачи поиска корреляционных зависимостей между спектральными параметрами комплексов с водородной связью и их геометрией, и энергией.
|
Tupikina et al. Phys. Chem. Chem. Phys. 2021 |
Karpov et al. J. Comput. Chem. 2021 |
Tupikina et al. |
Нами был предложен новый метод квантово-химической диагностики особенностей внешней электронной оболочки атомов, молекул и комплексов с водородной связью. Основания идея метода — использование атома 3He в качестве зондирующей частицы. Наша методика позволяет визуализировать как протоноакцепторные, так и протонодонорные свойства молекулярных систем и обладает преимуществами в сравнении с общепринятыми методами (MESP, ELF).
|
Tupikina et al. J. Phys. Chem. A 2017 |
Tupikina et al. J. Comput. Chem. 2018 |
Tupikina et al. J. Comput. Chem. 2020 |
Одним из направлений квантово-химических исследований нашей лаборатории является изучение особенностей электронного строения фрагментов биологических молекул (аминокислот, белков, ферментов). В частности, предсказание возможных направлений нуклеофильной атаки, изучение влияния дополнительных водородных связей с молекулами растворителя или боковыми цепями биологической молекулы на её реакционную способность.
Спектроскопия ЯМР
Метод ядерного магнитного резонанса (ЯМР) основан на регистрации резонансного поглощения энергии радиочастотного излучения образцом, помещенным в однородное магнитное поле. Излучение поглощается ядрами, имеющими ненулевой магнитный момент (1H, 13C, 19F, 31P и др.). Спектроскопия ЯМР является важнейшим методом определения структуры органических молекул.
В нашей лаборатории посредством экспериментальной спектроскопии ЯМР изучаются свойства различных систем с водородными и галогенными связями. В частности, мы ведём работу по разработке методики интерпретации экспериментальных значений химических сдвигов 31P как дескрипторов энергии и геометрии водородной связи. Установление количественных зависимостей между химическим сдвигом 31P осложняется (и потому становится более любопытным :)) по причине того, что ядро фосфора крайне чувствительно откликается на множество дополнительных факторов.
|
Mulloyarova et al. J. Molec. Struct. 2021 |
Giba et al. Symmetry 2021 |
Yakubenko et al. Phys. Chem. Chem. Phys, 2022 |
Для получения спектров ЯМР высокого разрешения при низких температурах (до 100 К) мы применяем и дорабатываем уникальную методику — в качестве растворителей используем смеси сжиженных газов фреонов. В таких условиях замедляются процессы протонного и молекулярного обмена и на спектрах ЯМР разрешаются сигналы комплексов различного стехиометрического и изотопного составов.
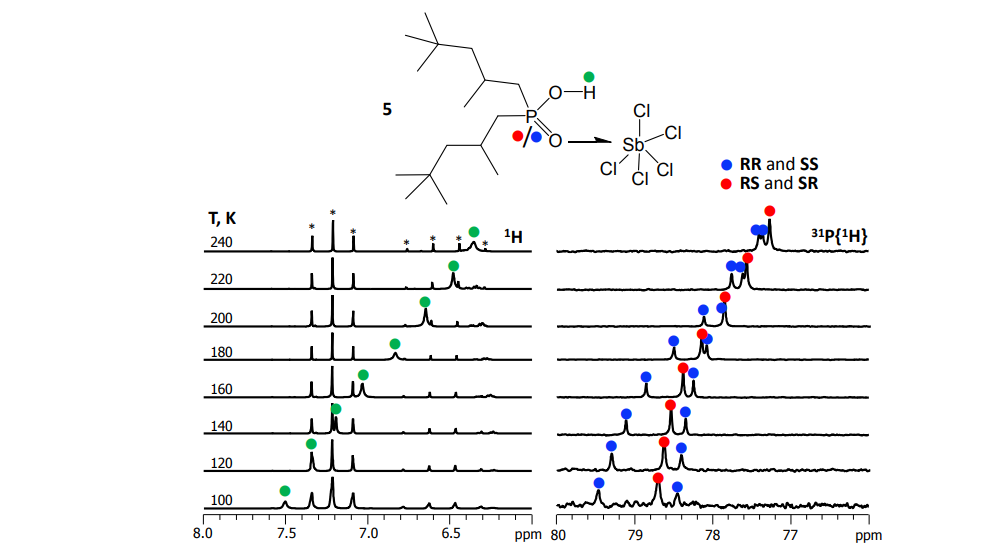
Mulloyarova et al. Phys. Chem. Chem. Phys. 2018
Наши гранты
- Грант РНФ 23-13-00095 «Нековалентные взаимодействия с высокой зарядовой кооперативностью» (руководительТолстойП.М.)
- Грант РНФ 24-73-10155 «Влияние динамики ядер на нековалентные взаимодействия: от фундаментального описания в модельных ассоциатах к предсказанию свойств супрамолекулярных систем» (руководительТупикинаЕ.Ю.)
- ГрантРНФ 24-73-00030 «С–Н-активация азиновых гетероциклов в условиях кооперативного катализа» (руководительТонкоглазоваД.И.).
Публикации
Избранные публикации 2020–2022 гг.
| M.A. Kostin, S.A. Pylaeva, P.M. Tolstoy, “Phosphine oxides as NMR and IR spectroscopic probes for the estimation of the geometry and energy of hydrogen bonds: PO…H-A hydrogen bonds”, Phys. Chem. Chem. Phys. 2022, 24, 7121-7133. DOI: 10.1039/D1CP05939D. | |
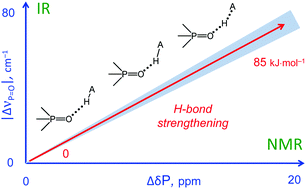 |
In this work we evaluate the possibility of using the NMR and IR spectral properties of the P O group to estimate the geometry and strength of hydrogen bonds which it forms with OH-, NH- and CH-acids. The results of the DFT study of 70 hydrogen-bonded 1 : 1 complexes of a model trimethylphosphine oxide, Me3PO, with various proton donors in the gas phase and in aprotic medium (modelled as a polarizable continuum) are presented. Four types of hydrogen bonds with the general formula Me3PO⋯H–A were considered, where the A atom is O, C, and N (neutral or cationic acids). Within the selected set of complexes the hydrogen bond energy varies over a wide range (ca. 0–85 kJ mol−1). We show that it is possible to use simple correlations to estimate the energy and geometry of OHO, NHO and CHO hydrogen bonds from the changes of isotropic 31P NMR chemical shifts and harmonic P O stretching vibration frequencies upon complexation. Such correlations also could be used to estimate the proton-donating ability (and Brønsted acidity; pKa) of OH acids. |
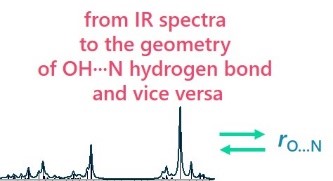
| E. Tupikina, A.A. Titova, M.V. Kaplanskiy, E.R. Chakalov, M.A. Kostin, P.M. Tolstoy, “Estimations of OH···N hydrogen bond length from positions and intensities of IR bands”, Spectrochimica Acta A 2022, published online DOI: 10.1016/j.saa.2022.121172 | |
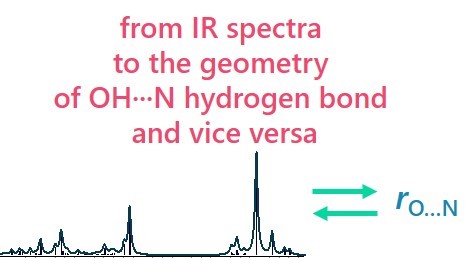 |
In this computational work applicability of IR spectral parameters for evaluations of OH···N hydrogen bond length is discussed. For a set of 124 complexes with OH···N hydrogen bond formed by combinations of methanol/acetic acid and pyridine (and their fluorine substituted versions) geometries, energies and IR parameters were calculated at MP2/def2-TZVP level of theory. For a number of IR parameters (the shift of proton donor group stretching vibration ∆ns, increase of its intensity I, the low-frequency hydrogen bond stretching vibration nσ, bending in-plane d and out-of-plane g vibrations) equations linking them with interatomic distances are proposed, the robustness and accuracy of such equations are discussed. The enthalpy of OH···N hydrogen bond formation ∆H was also linked with electron density parameters in (3; –1) critical point. |
| A. Yakubenko, A. Puzyk, V. Korostelev, V. Mulloyarova, E. Tupikina, P. Tolstoy, A. Antonov, “Oxidation of triphenylpnictogens and complexation of resulted di-phenylpnictoginic acids in solution and solid state”, Phys. Chem. Chem. Phys. 2022, published online. DOI: 10.1039/D2CP00286H. | |
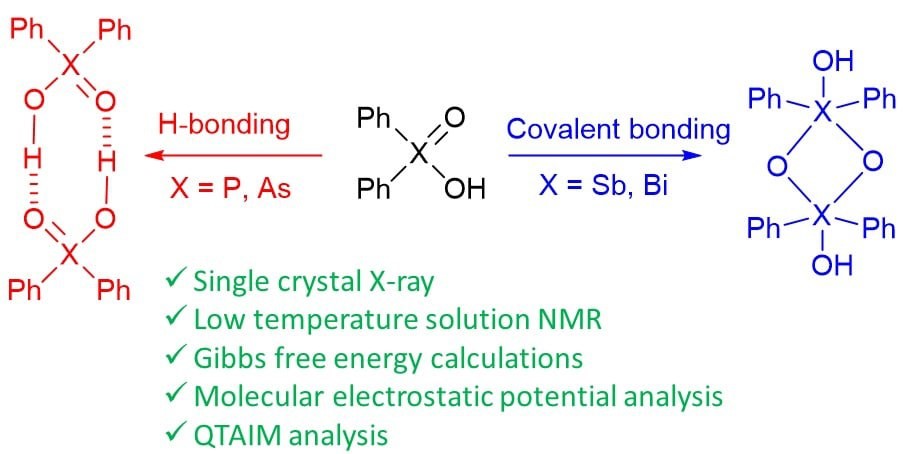 |
Triphenylpnictogens were oxidazed to access diphenylpnictiogenic acids Ph2XOOH (X = P, As, Sb, Bi). It was shown that oxidation with chloramine-T does not lead to the cleavage of a C–pnictogen bond. The preliminary reductive cleavage with sodium in liquid ammonia followed by the oxidation with hydrogen peroxide was successfully utilised for the synthesis of diphenylphosphinic and diphenylarsinic acids. It was shown that in solid state (by means of XRD), all diphenylpnictoginic acids form polymeric chains. Diphenylbismuthinic and diphenylantimonic acids form polymeric covalent adducts, while diphenylphosphinic and diphenylarsinic chains are associated through hydrogen bonding. Unlike diphenylphosphinic acid, diphenilarsinic acid forms two polymorphs of hydrogen-bonded infinite chains. In solution in a polar aprotic solvent diphenylarsinic acid, similarly to dimethylarsinic, forms hydrogen-bonded cyclic dimers together with a small amount of cyclic trimers. |
| A.A. Yakubenko, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “Lithiation of 2,4,5,7-Tetrabromo-1,8-bis(dimethylamino)naphthalene: Peculiarities of Directing Groups' Effects and the Possibility of Polymetalation”, Organometallics 2021, 40(21), 3627–36368. DOI: 10.1021/acs.organomet.1c00489. | |
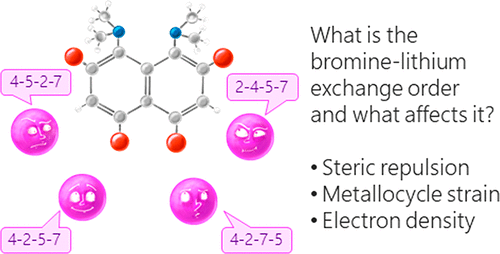 |
The bromine-lithium exchange sequence upon interaction of 2,4,5,7-tetrabromo-1,8-bis(dimethylamino)naphthalene with n-BuLi was studied. Experimental results were explained by means of quantum chemical calculations. It was demonstrated that the first exchange occurs in position 4 due to the significant decrease of a steric strain of the molecule. The second exchange takes place in either position 5 or 7 due to the even more negative charge distribution in the naphthalene core. As a result, the third exchange leads to the species containing lithium in positions 2,4,5 or 2,4,7. Using a large excess of n-BuLi in hexane, 2,4,5,7-tetralithio-1,8-bis(dimethylamino)naphthalene was successfully prepared. The latter was used for the synthesis of several tetrasubstituted derivatives of 1,8-bis(dimethylamino)naphthalene by quenching with different electrophiles. |
| B. Koeppe, J. Guo, P.M. Tolstoy, H.-H. Limbach, “NMR and UV-vis spectroscopic studies of models for the hydrogen bond system in the active site of photoactive yellow protein: hydrogen bond cooperativity and medium effects”, J. Phys. Chem. B 2021, 125, 22, 5874-5884. DOI: 10.1021/acs.jpcb.0c09923. | |
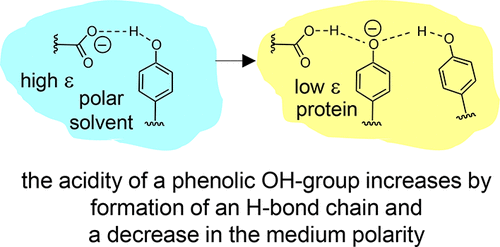 |
Intramolecular hydrogen bonds in aprotic media were studied by combined (simultaneous) NMR and UV–vis spectroscopy. The species under investigation were anionic and featured single or coupled H-bonds between, for example, carboxylic groups and phenolic oxygen atoms (COO···H···OC)−, among phenolic oxygen atoms (CO···H···OC)−, and hydrogen bond chains between a carboxylic group and two phenolic oxygen atoms (COO···H···(OC)···H···OC)−. The last anion may be regarded as a small molecule model for the hydrogen bond system in the active site of wild-type photoactive yellow protein (PYP) while the others mimic the corresponding H-bonds in site-selective mutants. Results of this work obtained from the model systems to PYP suggests that both cooperative effects within the hydrogen bond chain and a low-polarity protein environment are prerequisites for the stabilization of negative charge on the cofactor and hence for the spectral tuning of the photoreceptor. |
| I.S. Giba, P.M. Tolstoy, “Self-assembly of tetrahedral hydrogen-bonded cage tetramers of phosphonic acid”, Symmetry 2021, 13, 258. DOI: 10.3390/sym13020258. | |
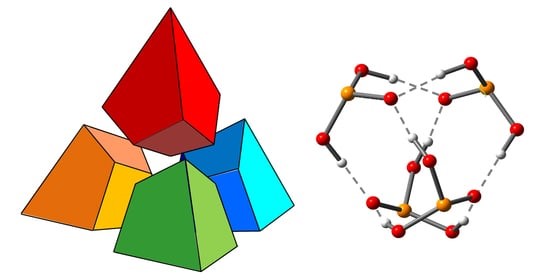 |
The self-association of phosphonic acids with general formula RP(O)(OH)2 in solution state remains largely unexplored. The general understanding is that such molecules form multiple intermolecular hydrogen bonds, but the stoichiometry of self-associates and the bonding motifs are unclear. In this work, we report the results of the study of self-association of tert-butylphosphonic acid using low temperature liquid-state 1H and 31P NMR spectroscopy (100 K; CDF3/CDF2Cl) and density functional theory (DFT) calculations. For the first time, we demonstrate conclusively that polar aprotic medium tert-butylphosphonic acid forms highly symmetric cage-like tetramers held by eight OHO hydrogen bonds, which makes the complex quite stable. In these associates. each phosphonic acid molecule is bonded to three other molecules by forming two hydrogen bonds as proton donor and two hydrogen bonds as proton acceptor. |
| V.V. Mulloyarova, A.M. Puzyk, A.A. Efimova, A.S. Antonov, R.A. Evarestov, I.S. Aliyarova, R.E. Asfin, P.M. Tolstoy, “Solid-State and Solution-State Self-Association of Dimethylarsinic Acid: IR, NMR and Theoretical Study”, J. Mol. Struct. 2021, 1234, 130176. DOI: 10.1016/j.molstruc.2021.130176. | |
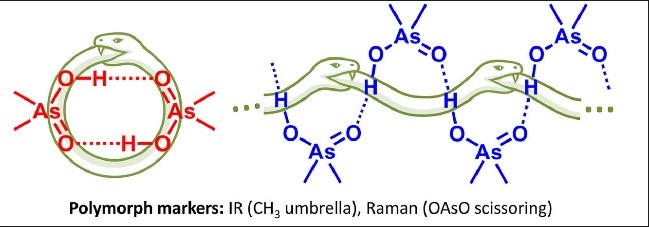 |
The structure of dimethylarsinic acid (Me2AsOOH, DMA) in crystal and in solution in aprotic medium was studied by X-ray, spectroscopic (IR, NMR and Raman) and quantum chemical calculation (DFT) methods. Two polymorphs of DMA - hydrogen-bonded cyclic dimers or infinite chains - were obtained by growing crystals from protic and aprotic solvents. By comparison of the experimental data with the results of quantum-chemical calculations performed with periodic boundary conditions we propose ways how to distinguish two polymorphs using IR marker bands. The self-association of DMA in polar aprotic solution was studied by low-temperature NMR (T = 100 K, solvent CDF3/CDF2Cl mixture). |
| E.Yu. Tupikina, V.V. Karpov, P.M. Tolstoy, “On the influence of water molecules on the outer electronic shells of R–SeH, R–Se(–) and R–SeOH fragments in selenocysteine amino acid residue”, Phys. Chem. Chem. Phys. 2021, 23, 13965-13970. DOI: 10.1039/D1CP01345A. | |
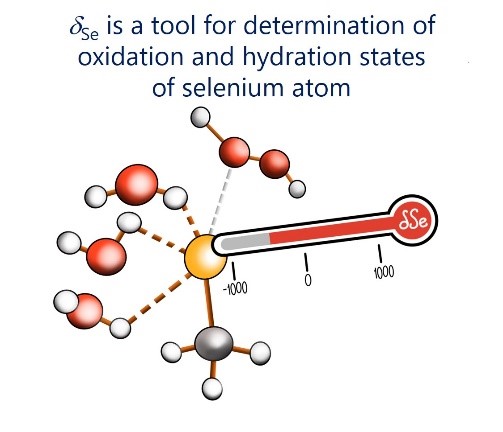 |
In this computational work (MP2/aug-cc-pVTZ) we investigated the features of the outer electronic shells of R–SeH, R–Se(−) and R–SeOH fragments (R = CH3), which can be considered as simplified models for the forms of the active centres of glutathione peroxidases GPx along their catalytic pathway (reduction of peroxides). It is shown that the preferential direction of a nucleophilic attack on the R–Se(−) fragment by a peroxide molecule is determined by the presence of the electron-depleted region of the selenium atom in front of the C–Se bond and nucleophilic attack can be facilitated by the solvation of R–Se(−) by water molecules. Such solvation does not block the direction of potential nucleophilic attack and also leads to the increase of the maximal value of the molecular electrostatic potential on the selenium atom. It was shown that the 77Se NMR chemical shift is sensitive both to the oxidation state and the hydration state of the selenium-containing fragment. |
| V. Karpov, A. Puzyk, P. Tolstoy, E. Tupikina, “Hydration of selenolate moiety: ab initio investigation of properties of O–H···Se(–) hydrogen bonds in CH3Se(–)∙∙∙(H2O)n clusters”, J. Comput. Chem. 2021, 42, 2014-2023. DOI: 10.1002/jcc.26733. | |
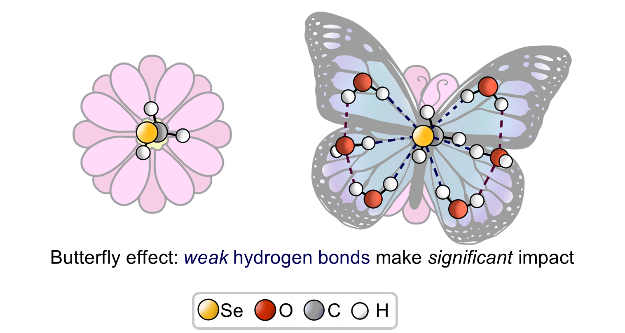 |
This work is devoted to investigations of the influence of O–H···Se(–) hydrogen bonds on the electronic shells of selenolate R–Se(–) fragment (R═CH3). The geometric, energetic and nuclear magnetic resonance (NMR) spectral parameters for various conformers of CH3Se(–)⋯(H2O)n clusters with n = 0–6 were calculated at CCSD/aug-cc-pVDZ level of theory. For selenolate anion CH3Se(–) solvation free energy was calculated, and for water media it is equal to −71.41 kcal/mol. For O–H···Se(–) hydrogen bonds the proportionality coefficients between QTAIM parameters at (3; −1) bond critical point and the strength of an individual hydrogen bond ∆E were proposed. It was shown, that despite a relative weakness of O–H···Se(–) hydrogen bonds, the outer electronic shell of the selenium atom changes significantly upon formation of each hydrogen bond. This, in turn, cause the dramatic change of NMR parameters of selenium nuclei. |
| E.Yu. Tupikina, P.M. Tolstoy, A.A. Titova, M.A. Kostin, G.S. Denisov, “Estimations of FH···X hydrogen bond energies from IR intensities: Iogansen's rule revisited”, J. Comput. Chem. 2021, 42(8), 564-571. DOI: 10.1002/jcc.26482 | |
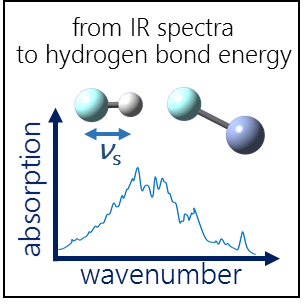 |
In this work the possibility of using the IR intensity of the stretching vibration νs of proton donor group for estimation of hydrogen bond strength was investigated. For a set of complexes with FH···X (X = F, N, O) hydrogen bonds in the wide range of energies (0.1–49.2 kcal/mol) vibrational frequencies νs and their intensities A were calculated (CCSD at complete basis set limit). The validity of the previously proposed linear proportionality between the intensification of the stretching vibration νs in IR spectra and hydrogen bond enthalpy –ΔH = 12.2·Δ√A (A.V.Iogansen, Spectrochimica Acta A 1999) was examined. It is shown that for a range of similar hydrogen bond types with complexation energies ∆E < 15 kcal/mol the ∆E(Δ√A) function remains similar to that proposed in the Iogansen’s work, while upon strengthening this dependency becomes significantly non-linear. We examined two other parameters (Δ√A/vs and Δ√A·mR) related to IR intensity as descriptors of hydrogen bond strength which are proportional to transition dipole moment matrix element and mass-independent dipole moment derivative. It was found that the dependency ∆E(Δ√A/vs) stays linear in the whole studied range of complexation energies and it can be used for evaluation of ∆E from infrared spectral data with the accuracy about 2 kcal/mol. The mass-independent product Δ√A·mR is an appropriate descriptor for sets of complexes with various hydrogen bond types. Simple equations proposed in this work can be used for estimations of hydrogen bond strength in various systems, where experimental thermodynamic methods or direct calculations are difficult or even impossible. |
| A.S. Antonov, V.V. Karpov, E.Yu. Tupikina, P.M. Tolstoy, M.A. Vovk, “Aggregation Behavior of Lithionaphthalenes in Solution: Experimental and Theoretical Study”, Organometallics 2020, 39, 3705. DOI: 10.1021/acs.organomet.0c00524 | |
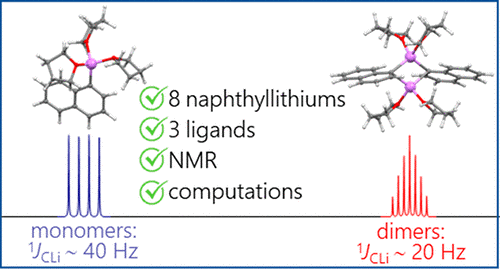 |
The aggregation of a series of mono- and dilithionaphthalenes in THF solutions in the presence of tetramethylethylenediamine (TMEDA) and pentamethyldiethylenetriamine (PMDTA) was studied by NMR spectroscopy and quantum chemical calculations. The stability of dimeric species in diluted solutions as well as reasons of their disaggregation in some cases has been discussed. For the lithioderivatives of 1,8-bis(dimethylamino)naphthalene (DMAN) the possibility of NMe2 group coordination to the lithium atom in ortho-position of naphthalene core under steric pressure of substituents was demonstrated by quantum chemical calculations. For the first time, dilithionaphthalenes were studied by 1H, 13C, and 7Li NMR spectroscopy. It was shown that while β-dilithionaphthalenes form oligomeric aggregates in solutions, α-dilithionaphthalenes exist as solely monomeric species with each lithium atom coordinated to both peri-positions. |
| E.Yu. Tupikina, K.G. Tokhadze, V.V. Karpov, G.S. Denisov, P.M. Tolstoy, “Stretching force constants as descriptors of energy and geometry of F···HF hydrogen bonds”, Spectrochim. Acta A 2020, 241, 118677. DOI: 10.1016/j.saa.2020.118677. | |
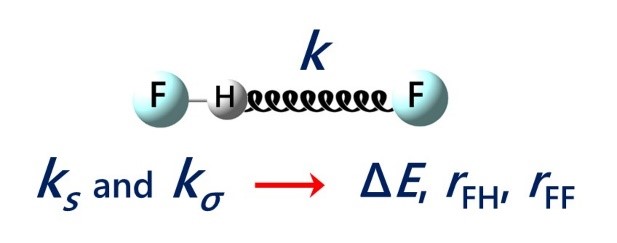 |
In this work applicability of proton donor group stretching vibration force constants ks and intermolecular stretching force constants kσ for evaluations of hydrogen bond strength and geometry are discussed. For a set of 30 complexes with F···HF hydrogen bonds in a wide range 0.5–48 kcal/mol by means of quantum chemical calculations equilibrium geometries, complexation energies, vibrational frequencies and corresponding force constants were calculated (MP2/aug-cc-pVTZ). It is shown, that properties of a hydrogen bond are more strictly correlated with the values of force constants than with vibrational frequencies. Easy-to-use equations for estimations of hydrogen bond energy ∆E and geometry (rFH, rFF) based on ks and kσ values are proposed. |
| A.S. Mazur, M.A. Vovk, P.M. Tolstoy, “Solid-state NMR of carbon nanostructures (graphite, graphene, carbon nanotubes, nanodiamonds, fullerene) in 2000-2019: a mini-review”, Fuller. Nanotub. Car. N. 2020, 28(3), 202-213. DOI: 10.1080/1536383X.2019.1686622. | |
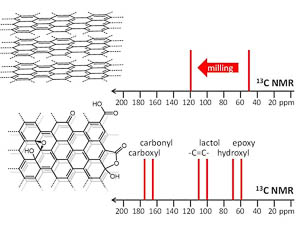 |
A brief overview is given of the papers published over the last two decades on the subject of solid-state 13C NMR spectra of such carbon nanostructures as milled graphite and its oxide, graphene, graphene oxide, carbon nanotubes, nanodiamonds, fullerenes and their derivatives. The main focus is put on the characteristic values of experimental isotropic 13C NMR chemical shifts and their interpretation in terms of local chemical environments of carbon nuclei. |
| V.V. Mulloyarova, D.O. Ustimchuk, A. Filarowski, P.M. Tolstoy, “H/D Isotope Effects on 1H NMR Chemical Shifts in Cyclic Heterodimers and Heterotrimers of Phosphinic and Phosphoric Acids”, Molecules 2020, 25, 1907. DOI: 10.3390/molecules25081907 | |
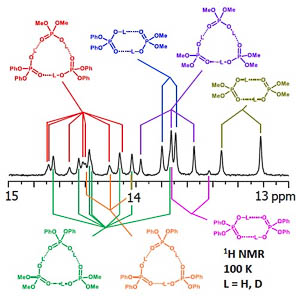 |
Hydrogen-bonded heterocomplexes formed by POOH-containing acids (diphenylphosphoric 1, dimethylphosphoric 2, diphenylphosphinic 3, and dimethylphosphinic 4) are studied by the low-temperature (100 K) 1H-NMR and 31P-NMR using liquefied gases CDF3/CDF2Cl as a solvent. Formation of cyclic dimers and cyclic trimers consisting of molecules of two different acids is confirmed by the analysis of vicinal H/D isotope effects (changes in the bridging proton chemical shift, δH, after the deuteration of a neighboring H-bond). Acids 1 and 4 (or 1 and 3) form heterotrimers with very strong (short) H-bonds (δH ca. 17 ppm). While in the case of all heterotrimers the H-bonds are cyclically arranged head-to-tail, ···O=P–O–H···O=P–O–H···, and thus their cooperative coupling is expected, the signs of vicinal H/D isotope effects indicate an effective anticooperativity, presumably due to steric factors: when one of the H-bonds is elongated upon deuteration, the structure of the heterotrimer adjusts by shortening the neighboring hydrogen bonds. We also demonstrate the formation of cyclic tetramers: in the case of acids 1 and 4 the structure has alternating molecules of 1 and 4 in the cycle, while in case of acids 1 and 3 the cycle has two molecules of 1 followed by two molecules of 3. |
| A.S. Ostras’, D.M. Ivanov, A.S. Novikov, P.M. Tolstoy, “Phosphine oxides as spectroscopic halogen bond descriptors: IR and NMR correlations with interatomic distances and complexation energy”, Molecules 2020, 25, 1406. DOI: 10.3390/molecules25061406 | |
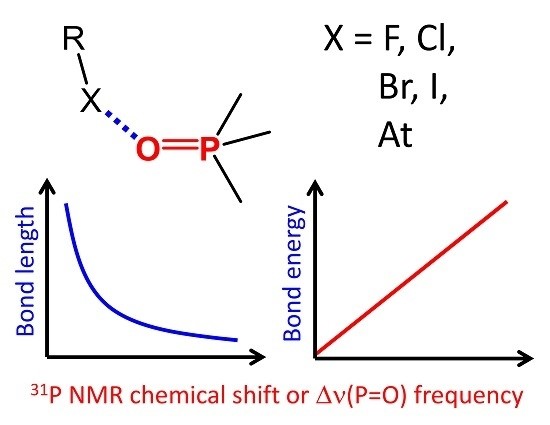 |
An extensive series of 128 halogen-bonded complexes formed by trimethylphosphine oxide and various F-, Cl-, Br-, I- and At-containing molecules, ranging in energy from 0 to 124 kJ/mol, is studied by DFT calculations in vacuum. The results reveal correlations between R–X···O=PMe3 halogen bond energy ∆E, X···O distance r, halogen’s σ-hole size, QTAIM parameters at halogen bond critical point and changes of spectroscopic parameters of phosphine oxide upon complexation, such as 31P NMR chemical shift, ∆δP, and P=O stretching frequency, ∆ν. Some of the correlations are halogen-specific, i.e., different for F, Cl, Br, I and At, such as ∆E(r), while others are general, i.e., fulfilled for the whole set of complexes at once, such as ∆E(∆δP). The proposed correlations could be used to estimate the halogen bond properties in disordered media (liquids, solutions, polymers, glasses) from the corresponding NMR and IR spectra. |
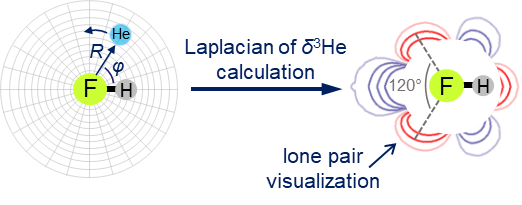
| E.Yu. Tupikina, K.G. Tokhadze, G.S. Denisov, P.M. Tolstoy, “Lone pairs mapping by Laplacian of 3He NMR chemical shift”, J. Comput. Chem. 2020, 41, 1194-1199. DOI: 10.1002/jcc.26166 | |
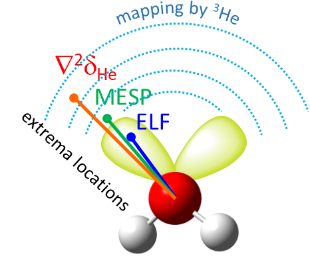 |
Results of approbation of a new quantum mechanical approach of lone pairs visualization, its optimization and testing on a range of model molecules are presented. The main idea of proposed methodology is using 3He atom as a probe for investigating electronic shells of species with lone pairs. As model objects we consider “classical” examples of hydrogen cyanide, methanimine, ammonia, phosphine, formaldehyde, water and hydrogen sulfide. It is shown, that lone pairs can be visualized by means of 3D maps of Laplacian of 3He chemical shift ∇2δHe. NMR calculations could be performed using level of theory as low as B3LYP/6-31G, allowing for the reduction of computational time without significant loss of quality. Advantages of our approach are discussed in comparison with usual methods of lone pairs visualization (ELF, MESP). |
| E. Yu. Tupikina, G. S. Denisov, A. S. Antonov, P. M. Tolstoy, “Unusual behaviour of the spin-spin coupling constant 1JCH upon formation of CH···X hydrogen bond”, Phys. Chem. Chem. Phys. 2020, 22, 1994-2000. DOI: 10.1039/C9CP05964D | |
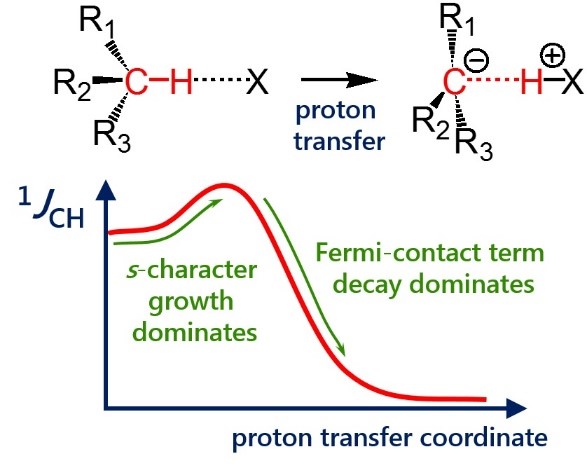 |
One-bond coupling constants 1JXY are usually used as a measure of corresponding X…Y interatomic distances. However the physical nature of this correlation is not well understood and in some cases a conterintuitive behaviour of 1JXY upon hydrogen bonded complex formation has been reported. In this work the behavior of 1JCH upon formation and strengthening of complexes with CH···X hydrogen bonds and upon proton transfer process is investigated by means of 1H NMR spectroscopy and quantum chemical calculations. 1H NMR spectra of 1,1-dinitroethane solution at room temperature in various solvents (carbon tetrachloride, chloroform, dichloromethane, acetone, dimethylformamide and dimethyl sulfoxide) illustrate the increase of 1JCH by several Hz upon increase of the complex strength. Computational results (MP2/aug-cc-pVDZ) reproduce this observation and allow one to conclude that the increase of 1JCH is mainly caused by the change of the carbon hybridization (an increase of s-character), rather than by the change in interatomic distance rCH. The behavior of 1JCH was also examined computationally in a wide range of CH···X hydrogen bonds’ energies and geometries. For this purpose quantum-chemical modeling of the partial proton transfer process for complexes formed by 1,1-dinitroethane and trinitromethane as hydrogen bond donors with acetone, pyridine and fluoride anion as hydrogen bond acceptors was performed. Obtained results have confirmed the abovementioned idea – for rather weak complexes the dominant impact on the change of 1JCH magnitude is the increase of the s-character of carbon atom hybridization, while for complexes with a significantly transferred proton the exponential decrease of the Fermi-contact term dominates. |
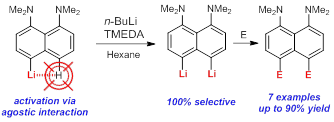
| A.A. Antonov, A.A. Yakubenko, “Noncovalent Li···H Interaction in the Synthesis of peri-Disubstituted Naphthalene Proton Sponges”, Synthesis 2020; 52, 98-104. DOI: 10.1055/s-0039-1690230 | |
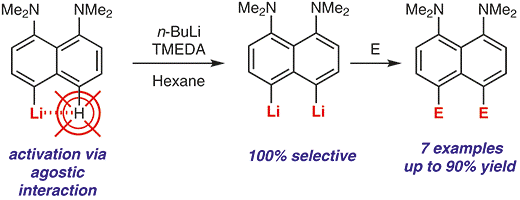 |
Noncovalent Li···H interaction was utilised as a tool for the second lithiation of 4-lithio-1,8-bis(dimethylamino)naphthalene with n-BuLi in the presence of TMEDA in hexane. Metallation proceeds with 100% selectivity in the second peri-position and with up to 90% yield. A series of 4,5-disubstituted derivatives of 1,8-bis(dimethylamino)naphthalene has been prepared in good to excellent yield after quenching the reaction mass with different electrophiles. |
| V. Mikshiev, A.F. Pozharskii, A. Filarowski, A.S. Novikov, A.S. Antonov, P.M. Tolstoy. M.A. Vovk, O.V. Khoroshilova, “How strong is hydrogen bonding to amide nitrogen?”, Chem. Phys. Chem. 2020, published online. DOI: 10.1002/cphc.201901104. | |
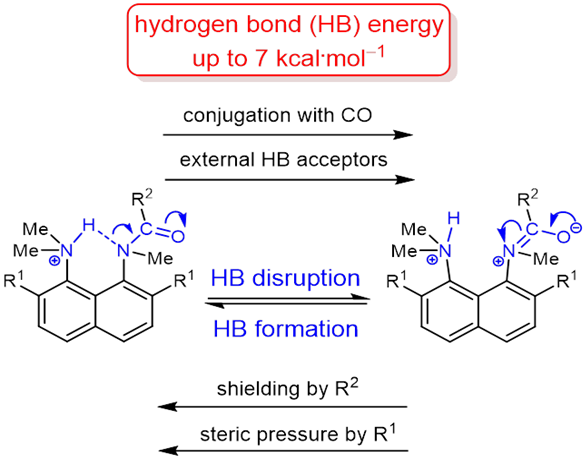 |
The protonation of the carboxamide nitrogen atom is an essential part of in vivo and in vitro processes (cis‐trans isomerization, amides hydrolysis etc). This phenomenon is well studied in geometrically strongly distorted amides, although there is little data concerning the protonation of undistorted amides. In the latter case, the participation of amide nitrogen in hydrogen bonding (which can be regarded as the incipient state of a proton transfer process) is less well‐studied. Thus, it would be a worthy goal to investigate the enthalpy of this interaction. We prepared and investigated a set of peri‐substituted naphthalenes containing the protonated dimethylamino group next to the amide nitrogen atom (“amide proton sponges”), which could serve as models for the study of an intramolecular hydrogen bond with the amide nitrogen atom. X‐Ray analysis, NMR spectra, basicity values as well as quantum chemical calculations revealed the existence of a hydrogen bond with the amide nitrogen, that should be attributed to the borderline between moderate and weak intramolecular hydrogen bonds (2–7 kcal·mol‒1). |
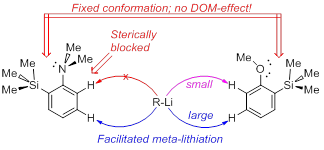
| A.S. Antonov, V.G. Bardakov, V.V. Mulloyarova, “Sterically facilitated meta-lithiation of arenes, containing electron-donating groups”, J. Org. Chem. 2020, 906, 121068. DOI: 10.1016/j.jorganchem.2019.121068 | |
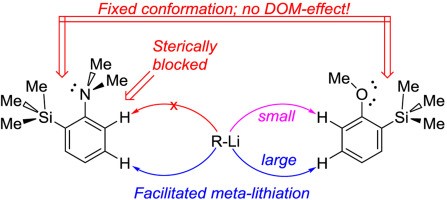 |
The influence of the bulky trimethylsilyl substituent on the selectivity of metallation of dimethylaniline, anisole and 1-dimethylaminonaphthalene is studied. The neighboring SiMe3 group forces dimethylamino and methoxy groups to occupy a conformation with an unshared electron pair turned towards silicon atom. This forced conformation prevents NMe2 and OMe groups from providing the DOM-effect, thus facilitating meta-metallation with the use of bulky LiCKOR. While the inverted NMe2 group completely suppresses ortho-metallation, the less bulky and more electron withdrawing OMe group demonstrates more rotation freedom allowing selective ortho-metallation with smaller reagents such as n-BuLi or tert-BuLi. |
Полный список публикаций лаборатории за 2024 г.
- D.A. Shitov, M.V. Kaplanskiy, E.Yu. Tupikina, « Influence of the Fe(II) Spin State on Iron-Ligand Bonds in Heme Model Iron-Porphyrin Complexes with 4, 5 and 6 Ligands», ChemPlusChem (2024). DOI: 10.1002/cplu.202400550.
- A.VRozhkov, E.Yu. Tupikina, K.I. Tugashov, V.Yu. Kukushkin, « Pure Heterometallic Spodium Bonding», CrystEngComm2024. DOI: 10.1039/D4CE00825A.
- A. Paderina, S. Slavova, E. Tupikina, D. Snetkov, E. Grachova, «Aggregation game: changing solid-state emission using different counterions in mono-alkynylphosphoniumPt(II) complexes », Inorganic Chemistry2024. DOI: 10.1021/acs.inorgchem.4c02130.
- A.S. Zakharov, D.V. Krutin, P.O. Mosalyov, E.Yu. Tupikina, A.S. Antonov, P.M. Tolstoy, V.V. Mulloyarova, “Phosphine Selenides: Versatile NMR Probes for Analyzing Hydrogen OH···Se and Halogen I···Se Bonds”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01895H
- M.V. Kaplanskiy, M.L. Kruglov, A.A. Vanin, E.Yu. Tupikina, “Dynamic of non-covalent interactions during the P–O bond cleavage by ribonuclease A”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01888E
- D.V. Krutin, A.S. Zakharov, E.Yu. Tupikina, V.V. Mulloyarova, “Unveiling the Electronic Structure Peculiarities of Phosphine Selenides as NMR Probes for Non-covalent Interactions: An Experimental and Theoretical Study”, Physical Chemistry Chemical Physics2024. DOI: 10.1039/D4CP01191K
- J. Eder, A.S. Antonov, E.Yu. Tupikina, R.M. Gschwind, “Chiral Diselenophosphoric Acids for Ion Pair Catalysis: A Novel Approach to Enhance Both Proton Donating and Proton Accepting Properties”, Chem. Eur. J. 2024, e202401793. DOI: 10.1002/chem.202401793
- E.Yu. Tupikina, M.V. Sigalov, O. Alkhuder, P.M. Tolstoy, “Charge Relay Without Proton Transfer: Coupling of Two Short Hydrogen Bonds via Imidazole in Models of Catalytic Triad of Serine Protease Active Site”, ChemPhysChem2024. DOI: 10.1002/cphc.202300970.
- D. A. Shitov, D. V. Krutin, E. Y. Tupikina, “Mutual influence of non-covalent interactions formed by imidazole: A systematic quantum-chemical study”, J. Comput. Chem.2024, 45(13), 1046-1060. DOI: 10.1002/jcc.27309.
- A.Yu. Samsonova, A.Yu. Mikheleva, K.M. Bulanin, N.I. Selivanov, A.S. Mazur, P.M. Tolstoy, C.C. Stoumpos, Y.V. Kapitonov, “Internal Vibrations of Pyridinium Cation in One-Dimensional Halide Perovskites and the Corresponding Halide Salts”, Molecules2024, 29, 78. DOI: 10.3390/molecules29010078.
- M.A. Kostin, O. Alkhuder, L. Xu, D.V. Krutin, R.E. Asfin, P.M. Tolstoy, “Complexes of phosphine oxides with substituted phenols: hydrogen bond characterization based on shifts of P=O stretching bands”, Phys. Chem. Chem. Phys.2024, 26, 10234-10242. DOI: 10.1039/D3CP05817D.
- S. Baykova, S. Baykov, K. Geyl, P. Tolstoy, V. Boyarskiy, “N-Pyridylureas as Masked Isocyanates for the Late-Stage Diversification of Pyridine-N-Oxides”, ChemistrySelect2024, 9, e202400720. DOI: 10.1002/slct.202400720.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, П.М. Толстой, «Структурная характеристика низкомолекулярного полибутадиена, синтезированного под действием катионной инициирующей системы», Изв. АН, серияхимическая2024, 73(4), 1035-1045.
- V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, P.M. Tolstoy, “Structural characterization of low molecular weight polybutadiene synthesized using cationic initiation system”, Russ. Chem. Bull.2024, 73(4), 1035-1045. DOI: 10.1007/s11172-024-4218-6.
- K. Sotiriadis, A.S. Mazur, Peter M. Tolstoy, P. Mácová, A. Viani, “External magnesium sulfate attack on hydrated Portland-limestone cements: Effect of the concurrent presence of sodium chloride in the corrosive environment and metakaolin admixture in the binder”, Cem. Concr. Compos.2024, 151, 105614. DOI: 10.1016/j.cemconcomp.2024.105614.
2022 г.
- A. Yakubenko, A. Puzyk, V. Korostelev, V. Mulloyarova, E. Tupikina, P. Tolstoy, A. Antonov, “Oxidation of triphenylpnictogens and complexation of resulted di-phenylpnictoginic acids in solution and solid state”, Phys. Chem. Chem. Phys. 2022, accepted. DOI: 10.1039/D2CP00286H.
- E. Tupikina, A.A. Titova, M.V. Kaplanskiy, E.R. Chakalov, M.A. Kostin, P.M. Tolstoy, “Estimations of OH···N hydrogen bond length from positions and intensities of IR bands”, Spectrochimica Acta A 2022, accepted
- M.A. Kostin, S.A. Pylaeva, P.M. Tolstoy, “Phosphine oxides as NMR and IR spectroscopic probes for the estimation of the geometry and energy of hydrogen bonds: PO…H-A hydrogen bonds”, Phys. Chem. Chem. Phys. 2022, 24, 7121-7133. DOI: 10.1039/D1CP05939D.
- Aliyarova, I.S., Tupikina, E.Yu., Ivanov, D.M., Kukushkin, V. Yu., “Metal-Involving Halogen Bonding Including Gold(I) as a Nucleophilic Partner. The Case of Isomorphic Dichloroaurate(I)Halomethane Cocrystals”, Inorganic Chemistry 2022, 61, 5, 2558–2567. DOI: 10.1021/acs.inorgchem.1c03482.
- K. Sotiriadis, K. Aspiotis, A. Mazur, P. Tolstoy, E. Badogiannis, S. Tsivilis, “Characterization of Old Concrete from a Heritage Structure of Inousses Cluster of Islands”, Lect. Notes Civ. Eng. 2022, 209, 80-89. DOI: 10.1007/978-3-030-90788-4_7.
- M. Nikishina, L. Perelomov, Y. Atroshchenko, E. Ivanova, L. Mukhtorov, P. Tolstoy, “Sorption of fulvic acids and their compounds with heavy metal ions on clay minerals”, Soil Syst. 2022, 6, 2. DOI: 10.3390/soilsystems6010002.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull. 2022, 79, 1239-1256. DOI: 10.1007/s00289-021-03549-5.
- F. Shakirova, U. Markova, P. Tolstoy, A. Bulatov, A. Shishov, “A new hydrophobic deep eutectic solvent based on thymol and 4-(dimethylamino)benzaldehyde: derivatization and microextraction of urea”, J. Mol. Liquids 2022, 353, 118820. DOI: 10.1016/j.molliq.2022.118820.
- A. Mazur, P. Tolstoy, K. Sotiriadis, “13С, 27Al and 29Si NMR investigation of the hydration kinetics of Portland-limestone cement pastes containing CH3-COO–R+ (R=H or Na)”, Materials 2022, 15, 2004. DOI: 10.3390/ma15062004.
- В.А. Розенцвет, Д.М. Ульянова, Н.А. Саблина, М.Г. Кузнецова, П.М. Толстой, «Полимеризация 1,3-пентадиена под действием катионных каталитических систем на основе алюминийорганических соединений», Известия АН 2022, 4, 787-795.
- V.A. Rozentsvet, D.M. Ulyanova, N.A. Sablina, S.V. Kostjuk, P.M. Tolstoy, I.A. Novakov, “Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes”, Polym. Chem. 2022, 3, 1596-1607. DOI: 10.1039/d1py01684a.
2021 г.
- A.A. Yakubenko, V.V. Karpov, E.Yu. Tupikina, A.S. Antonov, “Lithiation of 2,4,5,7-Tetrabromo-1,8-bis(dimethylamino)naphthalene: Peculiarities of Directing Groups' Effects and the Possibility of Polymetalation”, Organometallics 2021, 40(21), 3627–36368. DOI: 10.1021/acs.organomet.1c00489.
- V. Lyutoev, T. Shumilova, A. Mazur, P. Tolstoy, “NMR spectral characteristics of ultrahigh-pressure high-temperature impact glasses of the giant Kara astrobleme (Pay-Khoy, Russia)”, Minerals 2021, 11, 1418. DOI: 10.3390/min11121418.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, П.М. Толстой, И.А. Новаков, «Полимеризация изопрена под действием катионных каталитических систем на основе триэтилалюминия», Доклады РАН 2021, 499, 66-70. DOI: 10.31857/S2686953521040063. V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, P.M. Tolstoy, I.A. Novakov, “Polymerization of Isoprene Using Cationic Catalytic Systems Based on Triethylaluminum”, Doklady Phys Chem. 2021, 499, 73-76. DOI: 10.1134/S0012501621080017.
- E.Yu. Tupikina, P.M. Tolstoy, A.A. Titova, M.A. Kostin, G.S. Denisov, “Estimations of FH···X hydrogen bond energies from IR intensities: Iogansen's rule revisited”, J. Comput. Chem. 2021, 41. DOI: 10.1002/jcc.26482
- E.Y. Tupikina, S.G. Yastrebov, , “Molecular Complexes of Glycine with Cations H+, Ca2+, and Phosphine Oxide H3PO”, Technical Physics Letters 2021, 47(2), 147–149. DOI: 10.1134/S1063785021020140.
- I.S. Giba, P.M. Tolstoy, “Self-assembly of tetrahedral hydrogen-bonded cage tetramers of phosphonic acid”, Symmetry 2021, 13, 258. DOI: 10.3390/sym13020258.
- I.S. Giba, V.V. Mulloyarova, G.S. Denisov, P.M. Tolstoy, “Sensitivity of 31P NMR chemical shifts to hydrogen bond geometry and molecular conformation for complexes of phosphinic acids with pyridines”, Magn. Reson. Chem. 2020, accepted. DOI: 10.1002/mrc.5123.
- V.V. Mulloyarova, A.M. Puzyk, A.A. Efimova, A.S. Antonov, R.A. Evarestov, I.S. Aliyarova, R.E. Asfin, P.M. Tolstoy, “Solid-State and Solution-State Self-Association of Dimethylarsinic Acid: IR, NMR and Theoretical Study”, J. Mol. Struct. 2021, 1234, 130176. DOI: 10.1016/j.molstruc.2021.130176.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, С.Н. Смирнов, П.М. Толстой, «Строение полимерной цепи поли-1,3-пентадиена, синтезированного на стереоспецифической каталитической системе», Известия АН 2021, 4, 773-779. V.A. Rozentsvet, N.A. Sablina, D.M. Ulyanova, S.N. Smirnov, P.M. Tolstoy, “Structure of the polymer chain of poly(1,3-pentadiene) synthesized using a stereospecific catalytic system”, Russ. Chem. Bull. 2021, 70(4), 773-779. DOI: 10.1007/s11172-021-3150-2.
- B. Koeppe, J. Guo, P.M. Tolstoy, H.-H. Limbach, “NMR and UV-vis spectroscopic studies of models for the hydrogen bond system in the active site of photoactive yellow protein: hydrogen bond cooperativity and medium effects”, J. Phys. Chem. B 2021, 125, 22, 5874-5884. DOI: 10.1021/acs.jpcb.0c09923.
- Ł. Hetmańczyk, P. Szklarz, A. Kwocz, M. Wierzejewska, M. Pagacz-Kostrzewa, M.Ya. Melnikov, P.M. Tolstoy, A. Filarowski, “Polymorphism and Conformational Equilibrium for Nitro-Acetophenone in the Solid State and Under the Matrix Condition”, Molecules 2021, 26, 3109. DOI: 10.3390/molecules26113109.
- E.Yu. Tupikina, V.V. Karpov, P.M. Tolstoy, “On the influence of water molecules on the outer electronic shells of R–SeH, R–Se(–) and R–SeOH fragments in selenocysteine amino acid residue”, Phys. Chem. Chem. Phys. 2021, 23, 13965-13970. DOI: 10.1039/D1CP01345A.
- J. Savosina, M. Agafonova-Moroz, M. Khaydukova, A. Legin, V. Babain, P. Tolstoy, D. Kirsanov, “On the radiolytic stability of potentiometric sensors with plasticized polymeric membranes”, Chemosensors 2021, 9, 214. DOI: 10.3390/chemosensors9080214.
- E. Bulatov, T. Eskelinen, A. Ivanov, P. Tolstoy, E. Kalenius, P. Hirva, M. Haukka, “Noncovalent axial I∙∙∙Pt∙∙∙I interactions in platinum(II) complexes strengthen in the excited state”, ChemPhysChem 2021, 22, 1-7. DOI: 10.1002/cphc.202100468.
- Ł. Hetmańczyk, E.A. Goremychkin, J. Waliszewski, M.V. Vener, P. Lipkowski, P.M. Tolstoy, A. Filarowski, “Spectroscopic identification of hydrogen bond vibrations and quasi-isostructural polymorphism in N-salicylideneaniline”, Molecules 2021, 26, 5043. DOI: 10.3390/molecules26165043.
- V. Karpov, A. Puzyk, P. Tolstoy, E. Tupikina, “Hydration of selenolate moiety: ab initio investigation of properties of O–H···Se(–) hydrogen bonds in CH3Se(–)∙∙∙(H2O)n clusters”, J. Comput. Chem. 2021, 42, 2014-2023. DOI: 10.1002/jcc.26733.
- V. Rozentsvet, N. Sablina, D. Ulyanova, S. Kostjuk, P. Tolstoy, “Structure of terminal units of polybutadiene synthesized via anionic mechanism”, Polym. Bull. 2021, accepted. DOI: 10.1007/s00289-021-03549-5.
2020 г.
- K. Sotiriadis, P. Mácová, A.S. Mazur, A. Viani, P.M. Tolstoy, S. Tsivilis, “Long-term thaumasite sulfate attack on Portland-limestone cement concrete: A multi-technique analytical approach for assessing phase assemblage”, Cement and Concrete Research 2020, 130, 105995. DOI: 10.1016/j.cemconres.2020.105995.
- N.M. Kuznetsov, S.I. Belousov, A.V. Bakirov, S.N. Chvalun, R.A. Kamyshinsky, A.A. Mikhutkin, A.L. Vasiliev, P.M. Tolstoy, A.S. Mazur, E.D. Eidelman, E.B. Yudina, A.Ya. Vul', “Unique rheological behavior of detonation nanodiamond hydrosols: the nature of sol-gel transition”, Carbon 2020, 161, 486-494. DOI: 10.1016/j.carbon.2020.01.054.
- A.Yu. Baranov, A.S. Berezin, D.G. Samsonenko, A.S. Mazur, P.M. Tolstoy, V.F. Plyusnin, I.E. Kolesnikov, A.V. Artem'ev, “New Cu(I) halide complexes showing TADF combined with room temperature phosphorescence: the balance tuned by halogens”, Dalton Trans. 2020, 49, 3155-3163. DOI: 10.1039/D0DT00192A.
- I. Kritskiy, T. Volkova, S. Ivanov, A. Mazur, P. Tolstoy, I. Terekhova, “Methotrexate-Loaded Metal-Organic Frameworks on the Basis of γ-Cyclodextrin: Design, Characterization, in vitro and in vivo Investigation”, Materials Science and Engineering C 2020, 111, 10774. DOI: 10.1016/j.msec.2020.110774.
- В.А. Розенцвет, Н.А. Саблина, Д.М. Ульянова, М.Г. Кузнецова, С.В. Костюк, П.М. Толстой, «Идентификация строения терминальных звеньев полибутадиена методом ЯМР-спектроскопии с использованием Т2-фильтра», Доклады Академии Наук 2020, 491, 55-58. DOI: 10.31857/S2686953520020089. V.А. Rozentsvet, N.А. Sablina, D.M. Ulyanova, P.M. Tolstoy, S.N. Smirnov, I.A. Novakov, “Identification of the structure of polybutadiene terminal units by NMR spectroscopy with T2-filter”, Doklady Phys. Chem. 2020, 491, 40-42. DOI: 10.1134/S0012501620040028.
- V. Rozentsvet, N. Sablina, D. Ulyanova, M. Kuznetsova, S. Kostjuk, P. Tolstoy, “A new approach towards investigation of structure of terminal units of poly(1,3-pentadiene) using T2-filtered NMR spectroscopy”, Macromol. Chem. Phys. 2020, 2000168. DOI: 10.1002/macp.202000168.
- A.S. Antonov, V.V. Karpov, E.Yu. Tupikina, P.M. Tolstoy, M.A. Vovk, “Aggregation Behavior of Lithionaphthalenes in Solution: Experimental and Theoretical Study”, Organometallics 2020, 39, 3705. DOI: 10.1021/acs.organomet.0c00524.
- A.S. Antonov, E.Yu. Tupikina, V.V. Karpov, V.V. Mulloyarova, V.G. Bardakov, “Sterically Facilitated Intramolecular Nucleophilic NMe2 Group Substitution in the Synthesis of Fused Isoxazoles: Theoretical Study”, Molecules, 2020, 25, 5977. DOI: 10.3390/molecules25245977.
- A.S. Antonov, V.G. Bardakov, V.V. Mulloyarova, “Sterically facilitated meta-lithiation of arenes, containing electron-donating groups”, J. Org. Chem. 2020, 906, 121068. DOI: 10.1016/j.jorganchem.2019.121068
- A.A. Antonov, A.A. Yakubenko, “Noncovalent Li···H Interaction in the Synthesis of peri-Disubstituted Naphthalene Proton Sponges”, Synthesis 2020; 52, 98-104. DOI: 10.1055/s-0039-1690230
- E. Yu. Tupikina, G. S. Denisov, A. S. Antonov, P. M. Tolstoy, “Unusual behaviour of the spin-spin coupling constant 1JCH upon formation of CH•••X hydrogen bond”, Phys. Chem. Chem. Phys. 2020, 22, 1994-2000. DOI: 10.1039/C9CP05964D
- E. Yu. Tupikina, K. G. Tokhadze, G. S. Denisov, P. M. Tolstoy, “Lone pairs mapping by Laplacian of 3He NMR chemical shift”, J. Comput. Chem. 2020, 41, 1194-1199. DOI: 10.1002/jcc.26166.
- E.Yu. Tupikina, K.G. Tokhadze, V.V. Karpov, G.S. Denisov, P.M. Tolstoy, “Stretching force constants as descriptors of energy and geometry of F···HF hydrogen bonds”, Spectrochim. Acta A 2020, 241, 118677. DOI: 10.1016/j.saa.2020.118677.
- A.S. Mazur, M.A. Vovk, P.M. Tolstoy, “Solid-state NMR of carbon nanostructures (graphite, graphene, carbon nanotubes, nanodiamonds, fullerene) in 2000-2019: a mini-review”, Fuller. Nanotub. Car. N. 2020, 28(3), 202-213. DOI: 10.1080/1536383X.2019.1686622.
- A.S. Ostras’, D.M. Ivanov, A.S. Novikov, P.M. Tolstoy, “Phosphine oxides as spectroscopic halogen bond descriptors: IR and NMR correlations with interatomic distances and complexation energy”, Molecules 2020, 25, 1406. DOI: 10.3390/molecules25061406.
- V.Y. Mikshiev, A.F. Pozharskii, A. Filarowski, A.S. Novikov, A.S. Antonov, P.M. Tolstoy, M.A. Vovk, O.V. Khoroshilova, “ How strong hydrogen bond with amide nitrogen atom is?”, ChemPhysChem, 2020, 21, 651-658. DOI: 10.1002/cphc.201901104.
- V.V. Mulloyarova, D.O. Ustimchuk, A. Filarowski, P.M. Tolstoy, «H/D Isotope Effects on 1H NMR Chemical Shifts in Cyclic Heterodimers and Heterotrimers of Phosphinic and Phosphoric Acids», Molecules 2020, 25, 1907. DOI: 10.3390/molecules25081907.
- K. Jóźwiak, A. Jezierska, J.J. Panek, E.A. Goremychkin, P.M. Tolstoy, I.G. Shenderovich, A. Filarowski, “Inter- vs. intra-molecular hydrogen bond patterns and proton dynamics in phthalic acid associates”, Molecules 2020, 25, 4720. DOI: 10.3390/molecules25204720.
- V. Torres-Barthelemy, N. Pérez-Hernández, I.G. Shenderovich, P.M. Tolstoy, G.S. Denisov, H.-H. Limbach, “NMR-detected Host-Guest Proton Exchange as a novel Tool to study Surface/Volume Ratios and Filling of Cavities of mesoporous Solids”, J. Phys. Chem. C 2020, 124(40), 22082-22095. DOI: 10.1021/acs.jpcc.0c04889.
- P. Piękoś, A. Jezierska, J. Panek, E.A. Goremychkin, A. Pozharskii, A. Antonov, P. Tolstoy, A. Filarowski, “Symmetry/asymmetry of NHN hydrogen bond in protonated 1,8-bis(dimethylamino)naphthalene”, Symmetry 2020, 12, 1924. DOI: 10.3390/sym12111924.
- S.N. Yunusova, D.S. Bolotin, M.A. Vovk, P.M. Tolstoy, V.Yu. Kukushkin, “Tetrabromomethane as an Organic Catalyst: a Kinetic Study of CBr4-Catalyzed Schiff Condensation”, Eur. J. Org. Chem. 2020, 6763-6769. DOI: 10.1002/ejoc.202001180.
- D.L. Melnikova, Z.F. Badrieva, M.A. Kostin, C. Maller, M. Stas, A. Buczek, M.A. Broda, T. Kupka, A.-M. Kelterer, P.M. Tolstoy, V.D. Skirda, “On Complex Formation Between 5-Fluorouracil and beta-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR”, Molecules 2020, 25, 5706. DOI: 10.3390/molecules25235706.
2019 г.
- В.А. Розенцвет, В.Г. Козлов, О.А. Стоцкая, С.Н. Смирнов, П.М. Толстой, «Новый подход к изучению структуры полиизопрена, полученного методом катионной полимеризации», Изв. Акад. Наук 2019, 1, 116-120.
- M. Shevtsov, S. Stangl, B. Nikolaev, L. Yakovleva, Y. Marchenko, R. Tagaeva, W. Sievert, E. Pitkin, A. Mazur, P. Tolstoy, O. Galibin, V. Ryzhov, K. Steiger, W. Khachatryan, K.A. Chester, G. Multhoff, “Granzyme B functionalized nanoparticles targeting membrane Hsp70-positive tumors for multimodal cancer theranostics”, Small, 2019, 15, 1900205. DOI: 10.1002/smll.201900205.
- В.Е. Еремяшев, А.С. Мазур, П.М. Толстой, Л.М. Осипова, “Исследование особенностей структуры рубидиевых боросиликатных стекол методом ЯМР-спектроскопии ”, Неорганические материалы, 2019, 55, 538-543. DOI: 0.1134/S0002337X19050051.
- N.N. Sheveleva, D.A. Markelov, M.A. Vovk, M.E. Mikhailova, I.I. Tarasenko, P.M. Tolstoy, I.M. Neelov, E. Lähderanta, “Poly-L-lysine dendrimer with side arginine segments”, RSC Adv., 2019, 9, 18018. DOI: 10.1039/c9ra02461a.
- D. Lenz, B. Koeppe, P.M. Tolstoy, H.-H. Limbach, “An Efficient Perkin Synthesis of 13C-Labelled Cinnamic Acids From Acetic Acid as the Source of the Rare Isotope”, J. Label. Compd. Radiopharm., 2019, 62, 298-300. DOI: 10.1002/jlcr.3753.
- I.S. Giba, V.V. Mulloyarova, G.S. Denisov, P.M. Tolstoy, “Influence of Hydrogen Bonds in 1:1 Complexes of Phosphinic Acids with Substituted Pyridines on 1H and 31P NMR Chemical Shifts”, J. Phys. Chem. A 2019, 123, 2252-2260. DOI: 10.1021/acs.jpca.9b00625.
- E.Yu. Tupikina, M. Sigalov, I.G. Shenderovich, V.V. Mulloyarova, G.S. Denisov, P.M. Tolstoy, “Correlations of NHN hydrogen bond energy with geometry and 1H NMR chemical shift difference of NH protons for aniline complexes”, J. Chem. Phys. 2019, 150, 114305. DOI: 10.1063/1.5090180.
- V.V. Mulloyarova, I.S. Giba, G.S. Denisov, P.M. Tolstoy, “Conformational mobility and proton transfer in hydrogen-bonded dimers and trimers of phosphinic and phosphoric acids”, J. Phys. Chem A 2019, 123, 6761-6771. DOI: 10.1021/acs.jpca.9b05184.
- E.Yu. Tupikina, G.S. Denisov, P.M. Tolstoy, “Anticooperativity of FH···Cl– hydrogen bonds in [(FH)nCl]– clusters (n = 1…6)”, J. Comput. Chem. 2019, 40, 2858-2867. DOI: 10.1002/jcc.26066.
- A. F. Pozharskii, V. A. Ozeryanskii, V. Y. Mikshiev, A. V. Chernyshev, A. V. Metelitsa, A. S. Antonov, “Proton-induced fluorescence in modified quino[7,8-h]quinolines: dual sensing for protons and π-donors”, Org. Biomol. Chem., 2019, 17, 8221-8233. DOI: 10.1039/C9OB01391A.
- A. Jezierska, P. Tolstoy, J. Panek, A. Filarowski, “Intramolecular hydrogen bonds in selected aromatic compounds: recent developments”, Catalysts 2019, 9, 909. DOI: 10.3390/catal9110909.
- M.L. Khkhlov, A.E. Miroslavov, E.K. Legin, N.A. Korsakova, A.I. Kostylev, V.V. Gurzhiy, A.Yu. Ivanov, P.M. Tolstoi, “Tris(methyltrihydroborato)(tetrahydrofuran) ytterbium(iii) complex: structure and volatility”, Mendeleev Comm., 2019, 29, 696-697. DOI: 10.1016/j.mencom.2019.11.032.
- Ю.В. Шулевич, Ю.А. Захарова, П.М. Толстой, М.А. Вовк, Е.Г. Духанина, Д.С. Быков, А.В. Навроцкий, И.А. Новаков, «Матричная полимеризация триметилметакрилоилоксиэтиламмоний метилсульфата в мицеллярных растворах додецилсульфата натрия», Высокомолекулярные соединения, 2019, 61(6), 428-438. DOI: 10.1134/S2308113919060123.
Информация для студентов
Мы приглашаем в лабораторию студентов 1–4 курсов для выполнения выпускных квалификационных работ и работ по грантам по следующим направлениям:
- Спектроскопия ЯМР (проф. д.х.н.. Толстой П.М., пом. 2150, peter.tolstoy@spbu.ru)
- Квантовая химия (доц. к.ф.-м.н. Тупикина Е.Ю, пом. 2123)
- Элементоорганическая химия (к.х.н. Тонкоглазова Д.И.,daria.tonkoglazova@spbu.ru;к.х.н Цыбулин С.В.,s.tsybulin@spbu.ru,пом. 2135)