Научная группа профессора А.Ф. Хлебникова
Научная группа кафедры органической химии
Химия напряжённых азотсодержащих гетероциклов в функциональном молекулярном дизайне
198504, Санкт-Петербург, Петергоф, Университетский проспект, дом 26
Институт химии СПбГУ. Лаборатория № 4231
Тел. +7-812-3636000(9836); e-mail: a.khlebnikov@spbu.ru
Состав научной группы
Руководитель группы
Хлебников Александр Феодосиевич, д.х.н., профессор.
Состав группы
- Хлебников А. Ф., д.х.н., профессор
- Конев А. С., к.х.н., доцент;
- Галенко Е. Е., к.х.н., доцент,
- Занахов Т.О., аспирант,
- Таишев А. Э., студент,
- Матвеева Д. Р., студентка,
- Дудик А. С., студент,
- Ляхов Д. Д., студент
Тематика научной группы
I. Химия напряженных азотсодержащих гетероциклических соединений
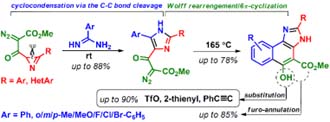
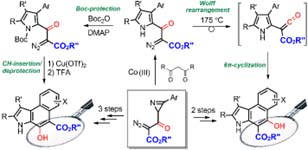
- Синтез и использование азиринов и изоксазолов в качестве молекулярных строительных блоков для синтеза новых гетероциклических систем и лигандов для комплексов металлов.
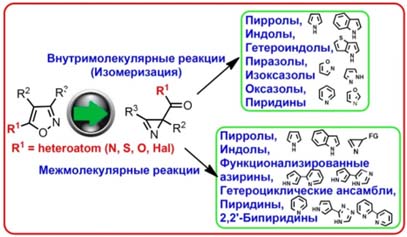
- N-илиды, металлокарбены, металлонитрены, радикалы и другие интермедиаты: генерирование и использование в гетероциклическом синтезе.
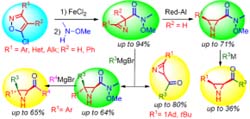
- Мультикатализ в синтезе гетероциклов.
II. Направленный синтез соединений с полезными свойствами на основе напряженных молекул:
- Поиск новых подходов к синтезу наноразмерных фуллеренсодержащих систем.
- Синтез гетероциклов с полезными фотофизическими свойствами, перспективных для использования в сенсорах и фотоэлектрических устройствах и эффективных в качестве лигандов для комплексов металлов.
III. Квантово-химические расчеты для понимания механизмов реакций, реакционной способности интермедиатов и рационального поиска реакционных партнеров.
Избранные публикации
 |
A. F. Khlebnikov, M. S. Novikov. Ring Expansions of Azirines and Azetines. Topics in Heterocyclic Chemistry, DOI: 10.1007/7081_2015_154, (Chapter in Synthesis of 4- To 7-Membered Heterocycles by Ring Expansion: Aza-, Oxa- And Thiaheterocyclic Small-Ring Systems, M.D. Hooghe,H.-J. Ha Eds, 2016, Springer; ISBN: 9783319249582; ISBN-10: 3319249584) |
 |
Khlebnikov A. F., Novikov M. S., Rostovskii N. V. Advances in 2H-azirine chemistry: A seven-year update. Tetrahedron 2019, 75, 2555-2624, DOI: 10.1016/j.tet.2019.03.040 |
 |
Funt L. D., Novikov M. S., Khlebnikov A. F. New applications of pyridinium ylides toward heterocyclic synthesis. Tetrahedron 2020, 76, 131415; DOI: 10.1016/j.tet.2020.131415. |
 |
V. N. Charushin, E. Verbitskiy, O. N. Chupakhin, et al. The chemistry of heterocycles in the 21st century. Russ. Chem. Rev., 2024, 93, RCR5125, DOI: 10.59761/RCR5125. |
Синтез и использование азиринов и изоксазолов в качестве молекулярных строительных блоков для синтеза новых гетероциклических систем и лигандов для комплексов металлов.
- Agafonova A. V., Novikov M. S., Khlebnikov A. F. Synthesis of 2H-azirine-2,2-dicarboxylic acids and their derivatives. Beilstein J. Org. Chem. 2024, 20, 3191–3197. DOI: 10.3762/bjoc.20.264.
- Prokop’eva, I. N.; Tomashenko, O. A.; Matveeva, D. R.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Azirine Weinreb amides: preparation and use in the synthesis of 2-acylated aziridines and azirines. Tetrahedron, 2024, 167, 134255, DOI: 10.1016/j.tet.2024.134255.
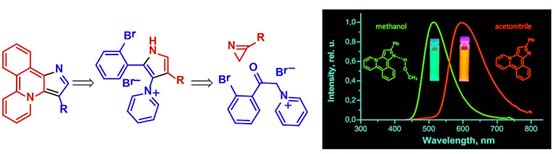
- Zanakhov, T.O., Galenko, E.E., Novikov, M.S., Khlebnikov, A.F. Cyclocondensation of 2‑(α-Diazoacyl)‑2H‑azirines with Amidines in Diazo Synthesis of Functionalized Naphtho[1,2‑d]imidazoles. J. Org. Chem. 2024, 89, 8641−8655. DOI: 10.1021/acs.joc.4c00598.
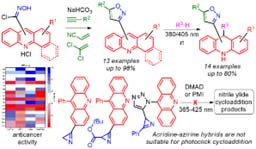
- Galenko, E. E.; Novikov, M. S.; Bunev, A. S.; Khlebnikov ,A. F. Acridine–Isoxazole and Acridine–Azirine Hybrids: Synthesis, Photochemical Transformations in the UV/Visible Radiation Boundary Region, and Anticancer Activity. Molecules 2024, 29, 1538. DOI: 10.3390/molecules29071538.
- Zanakhov, T. O.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Divergent Diazo Approach toward Alkyl 5/4- Hydroxy‑3H‑benzo[e]indole-4/5-carboxylates. J. Org. Chem. 2023, 88, 13191–13204. DOI: 10.1021/acs.joc.3c01413.
- Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. [2+ ] Cycloaddition/Retro-Electrocyclization/Decarboxylation Reaction Sequence: Access to 4‑Aminopyridines from Methylideneisoxazolones and Ynamines. J. Org. Chem. 2023, 88, 8854–8864. DOI: 10.1021/acs.joc.3c00654.
- Galenko, E. E.; Zanakhov, T. O.; Novikov, M. S.; Khlebnikov, A. F. Metal carbonyl mediated rearrangement of 5-(2-oxoalkyl)-1,2,4-oxadiazoles: Synthesis of fully substituted pyrimidines. Org. Biomol. Chem. 2023, 21, 2990-3001, DOI: 10.1039/D3OB00148B.
- Agafonova A. V., Novikov M. S., Khlebnikov A. F. 5-Chloroisoxazoles: A Versatile Starting Material for the Preparation of Amides, Anhydrides, Esters, and Thioesters of 2H-Azirine-2-carboxylic Acids. Molecules 2023, 28, 275; DOI: 10.3390/molecules28010275.
- Galenko, E. E.; Zanakhov, T. O.; Novikov, M. S.; Khlebnikov, A. F. Pd-Catalyzed Heteroannulation of Isoxazoles: Convergent Synthesis of Isoxazolo[5,4-c]quinolines. Tetrahedron Lett. 2023, 114, 154270, DOI: 10.1016/j.tetlet.2022.154270.
- Tomashenko, O. A.; Konev, A. S.; Khlebnikov, A. F. An Unusual Course of the Schmidt Rearrangement in the Reaction of 2H-Azirine-2-carbonyl Azides with Unsaturated Diazoesters. Russ. J. Gen. Chem. 2022, 92, 2197–2202, DOI: 10.1134/S1070363222100334.
- Zanakhov, T. O.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Diazo Strategy for Intramolecular Azirine Ring Expansion: Rh(II)-Catalyzed Synthesis of 2‑Hydroxy-3-oxo-2,3-dihydro‑1H‑pyrrole-2-carboxylates. J. Org. Chem. 2022, 87, 15598–15607. DOI: 10.1021/acs.joc.2c02177.
- Kaminskiy, N. A.; Galenko, E. E.; Kryukova, M. A.; Novikov, M. S.; Khlebnikov, A. F. Reaction of α‑Diazopyrroles with Enamines: Synthesis of Pyrrolo[2,1‑c][1,2,4]triazines and α‑(1,2,5-Triazapenta-1,3-dienyl)pyrroles. J. Org. Chem. 2022, 87, 10485−10492, DOI: 10.1021/acs.joc.2c01102.
- Krivolapova, Yu. V.; Tomashenko, O. A.; Funt, L. D.; Spiridonova, D. V.; Novikov, M. S.; Khlebnikov, A. F. Azirine-triazole hybrids: selective synthesis of 5-(2H-azirin-2-yl)-, 5-(1H-pyrrol-2-yl)-1H-1,2,3-triazoles and 2-(5-(2H-azirin-2-yl)-1H-1,2,3-triazol-1-yl)pyridines. Org. Biomol. Chem. 2022, 20, 5434–5443, DOI: 10.1039/D2OB00908K.
- Galenko, E. E.; Puzyk, A. M.; Novikov, M. S.; Khlebnikov, A. F. An Isoxazole Strategy for Molybdenum-Mediated Synthesis of 5‑Mono- and 4,5-Disubstituted 1H‑Pyrrole-2,3-diones. J. Org. Chem. 2022, 87, 6459−6470, DOI: 10.1021/acs.joc.2c00386.
- Zanakhov, T. O.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. An isoxazole strategy for the synthesis of 4-oxo-1,4-dihydropyridine-3-carboxylates. Beilstein J. Org. Chem. 2022, 18, 738–745. DOI: 10.3762/bjoc.18.74.
- Tomashenko, O. A.; Konev, A. S.; Khlebnikov, A. F. An Unusual Course of the Schmidt Rearrangement in the Reaction of 2H-Azirine-2-carbonyl Azides with Unsaturated Diazoesters. Russ. J. Gen. Chem. 2022, 92, 2197–2202, DOI: 10.1134/S1070363222100334.
- Efimenko N. I., Tomashenko O. A., Spiridonova D. V., Novikov M. S., Khlebnikov A. F. Nucleophile-Induced Rearrangement of 2H-Azirine-2-carbonyl Azides to 2-(1H-Tetrazol-1-yl)acetic Acid Derivatives. Org. Lett. 2021, 23, 6362–6366, DOI: 10.1021/acs.orglett.1c02157.
- Galenko, E. E.; Kaminskiy, N. A.; Novikov, M. S.; Khlebnikov, A. F. Synthesis of Water-Soluble α-Aminopyrroles, 1-(2-Amino-1H-pyrrol-3-yl)pyridinium Chlorides. Russ. J. Gen. Chem. 2021, 91, 1424-1428, DOI: 10.1134/S1070363221070239.
- Agafonova A. V., Funt L. D., Novikov M. S., Khlebnikov A. F. An isoxazole strategy for the synthesis of alkyl 5-amino-4-cyano-1H-pyrrole-2-carboxylates – versatile building blocks for assembling pyrrolo-fused heterocycles. Org. Biomol. Chem. 2021, 19, DOI: 10.1039/d1ob00053e.
- Galenko, E. E.; Bodunov, V. A.; Kryukova, M. A.; Novikov, M. S.; Khlebnikov, A. F. Buchner Reaction/Azirine Modification Approach Toward Cycloheptatriene Containing Nitrogen Heterocyclic Scaffolds. J. Org. Chem. 2021, 86, DOI: 10.1021/acs.joc.0c02928.
- Mosiagin, I. P.; Tomashenko, O. A.; Spiridonova, D. V.; Novikov, M. S.; Tunik, S. P.; Khlebnikov, A. F. Free-radical cyclization approach to polyheterocycles containing pyrrole and pyridine rings. Beilstein J. Org. Chem. 2021, 17, 1490–1498, DOI: 10.3762/bjoc.17.105;
- Zanakhov, T. O.; Galenko, E. E.; Kryukova, M. A.; Novikov, M. S.; Khlebnikov, A. F. Isomerization of 5-(2H-Azirin-2-yl)oxazoles: An Atom-Economic Approach to 4H-Pyrrolo[2,3-d]oxazoles. Molecules 2021, 26, 1881, DOI: 10.3390/molecules26071881;
- Galenko E. E., Yu. V. Kryukova M. A,. Novikov M. S, Khlebnikov A. F. Synthesis of Bi‑, Ter‑, and Quaterpyridinecarboxylates via Propargylisoxazole−Pyridine Rearrangement. J. Org. Chem. 2020, 85, 6109-6122, DOI: 10.1021/acs.joc.0c00611;
- Galenko E. E., Shakirova F. M., Bodunov V. A., Novikov M. S., Khlebnikov A. F. 1-(2H-Azirine-2-carbonyl)benzotriazoles: building blocks for the synthesis of pyrrole-containing heterocycles. Org. Biomol. Chem. 2020, 18, 2283–2296, DOI: 10.1039/D0OB00206B;
- Funt L. D., Krivolapova Yu. V., Khoroshilova O. V., Novikov M. S., Khlebnikov A. F. 2H-Azirine-2-carbonyl Azides: Preparation and Use as N-Heterocyclic Building Blocks. J. Org. Chem. 2020, 85, 4182-4194, DOI: 10.1021/acs.joc.9b03367;
- Galenko E. E., Linnik S. A., Khoroshilova O. V., Novikov M. S., Khlebnikov A. F. Isoxazole Strategy for the Synthesis of α-Aminopyrrole Derivatives. J. Org. Chem. 2019, 84, 11275-11285, DOI: 10.1021/acs.joc.9b01634;
- Bodunov V. A., Galenko E. E., Sakharov P.A., Novikov M. S., Khlebnikov A. F. Selective Cu-Catalyzed Intramolecular Annulation of 3-Aryl/Heteryl-2-(diazoacetyl)-1H-pyrroles: Synthesis of Benzo/Furo/Thieno[e]-Fused 1H-Indol-7-oles and Their Transformations. J. Org. Chem. 2019, 84, 10388-10401, DOI: 10.1021/acs.joc.9b01573;
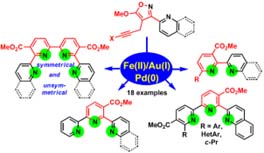
- Galenko E. E., Novikov M. S., Shakirova F. M., Shakirova J. R., Kornyakov I. V., Bodunov V. A., Khlebnikov A. F. An Isoxazole Strategy for the Synthesis of 2,2’-Bipyridine Ligands: Symmetrical and Unsymmetrical 6,6’-Binicotinates, 2,2’-Bipyridine-5-carboxylates, and Their Metal Complexes. J. Org. Chem. 2019, 84, 3524-3536, DOI: 10.1021/acs.joc.9b00115;
- Mikhailov K. I., Galenko E. E., Galenko A. V., Novikov M. S., Ivanov A. Yu., Starova G. L., Khlebnikov A. F. Fe(II)-Catalyzed isomerization of 5-chloroisoxazoles to 2H-azirine-2-carbonylchlorides as a key stage in the synthesis of pyrazole-nitrogen heterocycle dyads. J. Org. Chem. 2018, 83, 3177–3187; DOI: 10.1021/acs.joc.8b00069;
- Funt L. D., Tomashenko O. A., Novikov M. S., Khlebnikov A. F. An azirine strategy for the synthesis of alkyl 4-amino-5-(trifluoromethyl)-1H-pyrrole-2-carboxylates. Synthesis 2018, 50, 4809-4822; DOI: 10.1055/s-0037-1610840;
- Bodunov V. A., Galenko E. E., Galenko A. V., Novikov M. S., Khlebnikov A. F. Synthesis of Substituted Indole-3-carboxylates by Fe(II)-Catalyzed Domino Isomerization of 3-Alkyl/aryl-4-aryl-5-methoxyisoxazoles. Synthesis 2018, 50, 2784-2798, DOI: 10.1055/s-0036-1591576;
- Galenko E. E., Ivanov V. K., Novikov M. S., Khlebnikov A. F. Synthesis of N-aminopyrazoles by Fe(II)-catalyzed rearrangement of 4-hydrazonomethyl-substituted isoxazoles. Tetrahedron 2018, 74, 6288-6298; DOI: 10.1016/j.tet.2018.09.015;
- Sakharov P. A., Novikov M. S., Khlebnikov A. F. 2-Diazoacetyl-2H-azirines: Source of a Variety of 2H-Azirine Building Blocks with Orthogonal and Domino Reactivity. J. Org. Chem. 2018, 83, 8304-8314; DOI: 10.1021/acs.joc.8b01004;
- Galenko E. E., Bodunov V. A., Galenko A. V., Novikov M. S., Khlebnikov A. F. Fe(II)-Catalyzed Isomerization of 4-Vinylisoxazoles into Pyrroles. J. Org. Chem. 2017, 82, 8568−8579. DOI: 10.1021/acs.joc.7b01351;
- Galenko E. E., Khlebnikov, A. F.; Novikov, M. S. Isoxazole-azirine isomerization as a reactivity switch in the synthesis of heterocycles. Chem. Heterocycl. Compd. 2016, 52, 637–650. DOI: 10.1007/s10593-016-1944-1;
- Galenko E. E., Galenko A. V., Khlebnikov A. F., Novikov M. S., Shakirova J. R. Synthesis and intramolecular azo coupling of 4-diazopyrrole-2-carboxylates: selective approach to benzo and hetero [c]-fused 6H-pyrrolo[3,4-c]pyridazine-5-carboxylates. J. Org. Chem. 2016, 81, 8495−8507; DOI: 10.1021/acs.joc.6b01662;
- Galenko A. V., Khlebnikov A. F., Novikov M. S., Pakalnis V. V., Rostovskii N. V. Recent advances in isoxazole chemistry. Russ. Chem. Rev. 2015, 84, 335–377. DOI:10.1070/RCR4503;
- Galenko A. V., Khlebnikov A. F., Novikov M. S., Avdontseva M. S. Synthesis of 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-containing pyrroles. Tetrahedron 2015, 71, 1940–1951. DOI: 10.1016/j.tet.2015.02.030;
- Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Iakovenko, R. O.; Yufit D. S. Domino reactions of 2H-azirines with acylketenes from furan-2,3-diones: Competition between the formation of ortho-fused and bridged heterocyclic systems. Belst. J. Org. Chem. 2014, 10, 784-793. DOI:10.3762/bjoc.10.74;
- Khlebnikov, A. F.; Novikov, M. S. Recent advances in 2H-azirine chemistry. Tetrahedron 2013, 69, 3363-3401. DOI:10.1016/j.tet.2013.02.020,
N-илиды, металлокарбены, металлонитрены, радикалы и другие интермедиаты: генерирование и использование в гетероциклическом синтезе.
- Taishev, A. E.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Simple Access to Isoxazole-Containing Heterocyclic Hybrids: Isoxazole/Oxazole and Isoxazole/Pyridine. Russ. J. Gen. Chem. 2023, 93, 1246–1260, DOI: 10.1134/S1070363223050250.
- Galenko, E. E.; Bodunov, V. A.; Kryukova, M. A.; Novikov, M. S.; Khlebnikov, A. F. Buchner Reaction/Azirine Modification Approach Toward Cycloheptatriene Containing Nitrogen Heterocyclic Scaffolds. J. Org. Chem. 2021, 86, DOI: 10.1021/acs.joc.0c02928.
- Funt L. D., Krivolapova Yu. V., Khoroshilova O. V., Novikov M. S., Khlebnikov A. F. 2H-Azirine-2-carbonyl Azides: Preparation and Use as N-Heterocyclic Building Blocks. J. Org. Chem. 2020, 85, 4182-4194, DOI: 10.1021/acs.joc.9b03367;
- Galenko E. E., Shakirova F. M., Bodunov V. A., Novikov M. S., Khlebnikov A. F. 1-(2H-Azirine-2-carbonyl)benzotriazoles: building blocks for the synthesis of pyrrole-containing heterocycles. Org. Biomol. Chem. 2020, 18, 2283–2296, DOI: 10.1039/D0OB00206B;
- Galenko E. E., Linnik S. A., Khoroshilova O. V., Novikov M. S., Khlebnikov A. F. Isoxazole Strategy for the Synthesis of α-Aminopyrrole Derivatives. J. Org. Chem. 2019, 84, 11275-11285, DOI: 10.1021/acs.joc.9b01634;
- Bodunov V. A., Galenko E. E., Sakharov P.A., Novikov M. S., Khlebnikov A. F. Selective Cu-Catalyzed Intramolecular Annulation of 3-Aryl/Heteryl-2-(diazoacetyl)-1H-pyrroles: Synthesis of Benzo/Furo/Thieno[e]-Fused 1H-Indol-7-oles and Their Transformations. J. Org. Chem. 2019, 84, 10388-10401, DOI: 10.1021/acs.joc.9b01573;
- Funt L. D., Novikov M. S., Starova G. L., Khlebnikov A. F. Synthesis and properties of new heterocyclic betaines: 4-Aryl-5-(methoxycarbonyl)-2-oxo-3-(pyridin-1-ium-1-yl)-2,3-dihydro-1H-pyrrol-3-ides. Tetrahedron 2018, 74, 2466-2474, DOI: 10.1016/j.tet.2018.03.071;
- Kornilova T. A., Kostikov R. R., Khlebnikov A. F., Zenkevich I. G. Comparative characterization of thermal isomerization rates of 6‐phenyl‐1,5‐diazabicyclo[3.1.0]hexane at convection and microwave heating. J. Phys. Org. Chem. 2018, 31 (7), e3843; DOI: 10.1002/poc.3843;
- Funt L.D., Tomashenko O.A., Mosiagin I.P., Novikov M.S., Khlebnikov A.F. Synthesis of Pyrrolotriazoloisoquinoline Frameworks by Intramolecular Cu-Mediated or Free Radical Arylation of Triazoles. J. Org. Chem. 2017, 82, 7583-7594. DOI:10.1021/acs.joc.7b01341;
- Gaisina K. R., Khlebnikov A. F., Novikov M. S. Non-pericyclic cycloaddition of gem-difluorosubstituted azomethine ylides to the C=O bond: computational study and synthesis of fluorinated oxazole derivatives. Org. Biomol. Chem. 2017, 15, 4579-4586. DOI: 10.1039/C7OB00521K;
- Tomashenko O. A., Novikov M. S., Khlebnikov A. F. NHC as the Guiding Factor in a Copper-Catalyzed Intramolecular C Arylation of Pyrrolylimidazolium Salts: Synthesis of Luminescent Heterotetracyclic Frameworks. J. Org. Chem. 2017, 82, 616-623. DOI: 10.1021/acs.joc. 6b02627;
- Funt L. D., Tomashenko O. A., Khlebnikov A. F., Novikov M. S., Ivanov Yu. A. Synthesis, Transformations of Pyrrole- and 1,2,4-Triazole-Containing Ensembles, and Generation of Pyrrole-Substituted Triazole NHC. J. Org. Chem. 2016, 81, 11210-11221. DOI: 10.1021/acs.joc.6b02200;
- Khlebnikov A. F., Novikov M. S., Gorbunova Y. G., Galenko E. E., Mikhailov K. I., Pakalnis V. V., Avdontceva M. S. Isoxazolium N-ylides and 1-oxa-5-azahexa-1,3,5-trienes on the way from isoxazoles to 2H-1,3-oxazines. Belst. J. Org. Chem. 2014, 10, 1896-1905. DOI:10.3762/bjoc.10.197;
- Khlebnikov A. F., Tomashenko O. A., Funt L. D., Novikov M. S. Simple Approach to Pyrrolylimidazole Derivatives by Azirine Ring Expansion with Imidazolium Ylides. Org. Biomol. Chem. 2014, 12, 6598-6609. DOI: 10.1039/c4ob00865k;
- Khlebnikov A. F., Konev A. S., Virtsev A. A., Yufit D. S., Mlostoń G., Heimgartner H. Concerted vs. Non-Concerted 1,3-Dipolar Cycloadditions of Azomethine Ylides to Electron-Deficient Dialkyl 2,3-Dicyanobut-2-enedioates. Helv. Chim. Acta. 2014, 97, 453-470. DOI:10.1002/hlca.201300405,
Мультикатализ в синтезе гетероциклов.
- Galenko E. E., Yu. V. Kryukova M. A,. Novikov M. S, Khlebnikov A. F. Synthesis of Bi‑, Ter‑, and Quaterpyridinecarboxylates via Propargylisoxazole−Pyridine Rearrangement. J. Org. Chem. 2020, 85, 6109-6122, DOI: 10.1021/acs.joc.0c00611;
- Bodunov V. A., Galenko E. E., Sakharov P.A., Novikov M. S., Khlebnikov A. F. Selective Cu-Catalyzed Intramolecular Annulation of 3-Aryl/Heteryl-2-(diazoacetyl)-1H-pyrroles: Synthesis of Benzo/Furo/Thieno[e]-Fused 1H-Indol-7-oles and Their Transformations. J. Org. Chem. 2019, 84, 10388-10401, DOI: 10.1021/acs.joc.9b01573;
- Galenko E. E., Novikov M. S., Shakirova F. M., Shakirova J. R., Kornyakov I. V., Bodunov V. A., Khlebnikov A. F. An Isoxazole Strategy for the Synthesis of 2,2’-Bipyridine Ligands: Symmetrical and Unsymmetrical 6,6’-Binicotinates, 2,2’-Bipyridine-5-carboxylates, and Their Metal Complexes. J. Org. Chem. 2019, 84, 3524-3536, DOI: 10.1021/acs.joc.9b00115;
- Funt L. D., Tomashenko O. A., Novikov M. S., Khlebnikov A. F. An azirine strategy for the synthesis of alkyl 4-amino-5-(trifluoromethyl)-1H-pyrrole-2-carboxylates. Synthesis 2018, 50, 4809-4822, DOI: 10.1055/s-0037-1610840;
- Galenko E. E., Ivanov V. K., Novikov M. S., Khlebnikov A. F. Synthesis of N-aminopyrazoles by Fe(II)-catalyzed rearrangement of 4-hydrazonomethyl-substituted isoxazoles. Tetrahedron 2018, 74, 6288-6298, DOI: 10.1016/j.tet.2018.09.015;
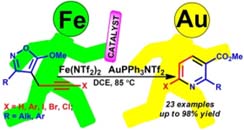
- Galenko A. V., Galenko E. E., Shakirova F. M., Novikov M. S., Khlebnikov A. F. Fe(II)/Au(I) Relay Catalyzed Propargylisoxazole to Pyridine Isomerization: Access to 6-Halonicotinates. J. Org. Chem. 2017, 82, 5367-5379. DOI: 10.1021/acs.joc.7b00736;
- Galenko E. E., Tomashenko O. A., Khlebnikov A. F., Novikov M. S. Fe(II)/Et3N-Relay-catalyzed domino reaction of isoxazoles with imidazolium salts in the synthesis of methyl 4-imidazolylpyrrole-2-carboxylates, its ylide and betaine derivatives. Beilstein J. Org. Chem. 2015, 11, 1732–1740. DOI:10.3762/bjoc.11.189;
- Galenko E. E., Tomashenko O. A., Khlebnikov A. F., Novikov M. S. Metal/organo relay catalysis in a one‐pot synthesis of methyl 4-aminopyrrole‐2‐carboxylates from 5‐methoxyisoxazoles and pyridinium ylides. Org. Biomol. Chem. 2015, 13, 9825-9833; DOI: 10.1039/c5ob01537e;
- Galenko E. E., Galenko A. V., Khlebnikov A. F., Novikov M. S. Domino transformation of isoxazoles to 2,4-dicarbonylpyrroles under Fe/Ni relay catalysis. RSC Advances 2015, 5, 18172-18176. DOI: 10.1039/C5RA01889G.
Синтез и использование азиридинов как эффективных источников активных интермедиатов для построения сложных молекулярных систем.
- Androsov D. V, Konev A. S., Khlebnikov A. F. Aziridine strategy for stereospecific synthesis of 1′-alkyl/aryl-5′-aryl-2′,5′-dihydropyrrolofullerene-2′-carboxylates and NMR study of hindered 5′-aryl group rotation. Tetrahedron 2022, 78, 132734, DOI: 10.1016/j.tet.2022.132734.
- Kazakova A. V., Androsov D. V., Konev A. S., Khlebnikov A. F. Magnesium acetate – an effective electrophilic activator of the carbonyl group in transesterification of dialkylaziridine dicarboxylates. Chem. Heterocycl. Compd. 2020, 56, 875–880, DOI: 10.1007/s10593-020-02744-y.
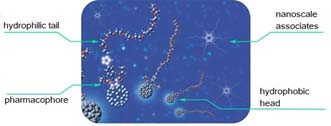
- Kazakova A. V., Konev A. S., Zorin I. M., Poshekhonov V. A., Korzhikov-Vlakh V. A., Khlebnikov A. F. PEG-modified aziridines for stereoselective synthesis of water-soluble fulleropyrrolidines. Org. Biomol. Chem. 2019, 17, 9864–9873, DOI: 10.1039/c9ob01949a;
- Lukyanov D.A., Funt L.D., Konev A.S., Povolotskiy A. V., Vereshchagin A. A., Levin O.V., Khlebnikov A.F. Novel homogeneous photocatalyst for oxygen to hydrogen peroxide reduction in aqueous media. Photochem. Photobiol. Sci. 2019, 18, 1982-1989, DOI: 10.1039/C9PP00206E;
- Androsov D. V., Strelnikov A. A., Konev A. S., Lukyanov D. A., Kazakova A. V., Levin O. V., Khlebnikov A. F. Photogalvanic effect in porphyrin-pyrrolo[3´,4´:1,9]-(C60-Ih)[5,6]fullerene-2´,5´-dicarboxylate systems. Russ. Chem. Bull. 2019, 68, 825-831, DOI: 10.1007/s11172-019-2491-6;
- Lukyanov D. A., Konev A. S., Amsharov K. Yu., Khlebnikov A. F., Hirsch A. Diastereospecific and highly site-selective functionalization of C70 fullerene by reaction with diethyl N-arylaziridine-2,3-dicarboxylates. J. Org. Chem. 2018, 83, 14146-14151, DOI: 10.1021/acs.joc.8b02240;
- Strelnikov A. A., Androsov D. V., Konev A. S., Lukyanov D. A., Khlebnikov A. F., Povolotskiy A. V., Yamanouchi K. Triaryl-substituted pyrrolo-p-phenylene-linked porphyrin-fullerene dyads: Expanding the structural diversity of photoactive materials. Tetrahedron 2018, 74, 3007-3019, DOI: 10.1016/j.tet.2018.04.084;
- Lukyanov, D. A.; Konev, A. S.; Amsharov, K. Yu.; Khlebnikov, A. F.; Hirsch A. Diastereospecific and highly site-selective functionalization of C70 fullerene by reaction with diethyl N-arylaziridine-2,3-dicarboxylates. J. Org. Chem., 2018, 83, 14146-14151; DOI: 10.1021/acs.joc.8b02240;
- Strelnikov A. A., Androsov D. V., Konev A. S., Lukyanov D. A., Khlebnikov A. F., Povolotskiy A. V., Yamanouchi K. Triaryl-substituted pyrrolo-p-phenylene-linked porphyrin-fullerene dyads: Expanding the structural diversity of photoactive materials. Tetrahedron 2018, 74, 3007-3019, DOI: 10.1016/j.tet.2018.04.084;
- Konev A. S., Khlebnikov A. F., Levin O. V., Lukyanov D. A., Zorin I. M. Photocurrent in Multilayered Assemblies of Porphyrin–Fullerene Covalent Dyads: Evidence for Channels for Charge Transport. ChemSusChem. 2016, 9, 676–686. DOI:10.1002/cssc.201501686;
- Konev A. S., Khlebnikov A. F., Prolubnikov P. I., Mereshchenko A. S., Povolotskiy A. V., Levin O. V., Hirsch A. Synthesis of New Porphyrin–Fullerene Dyads Capable of Forming Charge-Separated States on a Microsecond Lifetime Scale. Chem. Eur. J. 2015, 21, 1237–1250. DOI:10.1002/chem.201404435;
- Konev A. S., Lukyanov D. A., Vlasov P. S., Levin O. V., Virtsev A. A., Kislyakov I. M.; Khlebnikov A. F. The Implication of 1,3-Dipolar Cycloaddition of Azomethine Ylides to the Synthesis of Main-Chain Porphyrin Oligomers. Macromol. Chem. Phys. 2014, 215, 516-529. DOI: 10.1002/macp.201300679;
- Konev A. S., Khlebnikov A. F., Nikiforova T. G., Virtsev A. A., Frauendorf H. Synthesis and spectroscopic and electrochemical properties of an axially symmetric fullerene-porphyrin dyad with a rigid pyrrolo[3,4-c]pyrrole spacer. J. Org. Chem. 2013, 78, 2542-2552. DOI: 10.1021/jo302763a;
Направленный синтез соединений с полезными свойствами на основе напряженных молекул.
- Vidyakina A. A.; Shtyrov A. A.; Ryazantsev M. N., Khlebnikov A. F., Kolesnikov I. E.; Sharoyko V. V., Spiridonova D. V.; Balova I. A.; Bräse S., Danilkina N. A. Development of Fluorescent Isocoumarin-Fused Oxacyclononyne ‒ 1,2,3-Triazole Pairs. . Chem. Eur. J., 2023, 29, e202300540, DOI: 10.1002/chem.202300540.
- Kuznetsov, K. M.; Baigildin, V. A.; Solomatina, A. I.; Galenko, E. E.; Khlebnikov, A. F.; Sokolov, N. V.; Tunik, S. P.; Shakirova, J. R Polymeric nanoparticles with embedded Eu(III) complexes as molecular probes for temperature sensing. Molecules 2022, 27, 8813; DOI: 10.3390/molecules27248813.
- Solomatina A. I., Galenko E. E., Kozina D. O., Kalinichev A. A., Baigildin V. A., Prudovskaya N. A., Shakirova J. R., Khlebnikov A. F., Porsev V. V., Evarestov R. A., Tunik S. P. Non-symmetric [Pt(C^N*N′^C′)] complexes: aggregation induced emission in solid state and in nanoparticles tuned by ligand structure. Chem. Eur. J., 2022, 28, e202202207, DOI: 10.1002/chem.202202207.
- Zharskaia, N. A.; Solomatina, A. I.; Liao, Y.-C.; Galenko, E. E., Khlebnikov, A. F.; Chou, P.-T.; Chelushkin, P. S.; Tunik, S. P. Aggregation-Induced Ignition of Near-Infrared Phosphorescence of Non-Symmetric [Pt(CˆN*N’ˆC’)] Complex in Poly(caprolactone)-based Block Copolymer Micelles: Evaluating the Alternative Design of Near-Infrared Oxygen Biosensors. Biosensors 2022, 12, 695; DOI: 10.3390/bios12090695.
- Kazakova A. V., Rubicheva L. G., Konev A. S., Khlebnikov A. F. Stereoselective synthesis of PEGylated azoles via 1,3-dipolar cycloaddition. Tetrahedron 2021, 77, 131774, DOI: 10.1016/j.tet.2020.131774.
- Kozina D. O., Shakirova J. R., Galenko E. E., Porsev V. V., Gurzhiy V. V., Khlebnikov A. F., Tunik S. P. Unusual Reactivity and Photophysical Properties of Platinum(II) Pincer Complexes Containing 6,6'‐Diphenyl‐2,2'‐bipyridine Ligands. Europ. J. Inorg. Chem. 2021, 117-125, DOI: 10.1002/ejic.202000827.
- Strelnikov A. A., Konev A. S., Levin O. V., Khlebnikov A. F., Iwasaki, A.; Yamanouchi, K.; Tkachenko, N. V. Switching competition between electron and energy transfers in porphyrin-fullerene dyads. J. Phys. Chem. B 2020, 124, 10899–10912, DOI: 10.1021/acs.jpcb.0c06931.
- Plass F., Lukyanov D. A., Konev A. S., Kahnt A., Amsharov K. Y., Khlebnikov A. F., Guldi D. M. Controlling the Charge Transfer Mechanism and Efficiency by Means of Different C70 Regioisomeric Adducts. Small Structures 2020, 1, 2000012, DOI: 10.1002/sstr.202000012.
- Galenko E. E., Yu. V. Kryukova M. A,. Novikov M. S, Khlebnikov A. F. Synthesis of Bi‑, Ter‑, and Quaterpyridinecarboxylates via Propargylisoxazole−Pyridine Rearrangement. J. Org. Chem. 2020, 85, 6109-6122, DOI: 10.1021/acs.joc.0c00611;
- Shakirova J. R., Shevchenko N. N., Baigildin V. A., Chelushkin. P. S., Khlebnikov A. F., Tomashenko O. A., Solomatina A. I., Starova G. L., Tunik S. P. Eu-Based Phosphorescence Lifetime Polymer Nanothermometer: A Nanoemulsion Polymerization Approach to Eliminate Quenching of Eu Emission in Aqueous Media. ACS Appl. Polym. Mater. 2020, 2, 537-547, DOI: 10.1021/acsapm.9b00952;
- Kazakova A. V., Konev A. S., Zorin I. M., Poshekhonov V. A., Korzhikov-Vlakh V. A., Khlebnikov A. F. PEG-modified aziridines for stereoselective synthesis of water-soluble fulleropyrrolidines. Org. Biomol. Chem. 2019, 17, 9864–9873, DOI: 10.1039/c9ob01949a;
- Lukyanov D.A., Funt L.D., Konev A.S., Povolotskiy A. V., Vereshchagin A. A., Levin O.V., Khlebnikov A.F. Novel homogeneous photocatalyst for oxygen to hydrogen peroxide reduction in aqueous media. Photochem. Photobiol. Sci. 2019, 18, 1982-1989, DOI: 10.1039/C9PP00206E;
- Bodunov V. A., Galenko E. E., Sakharov P.A., Novikov M. S., Khlebnikov A. F. Selective Cu-Catalyzed Intramolecular Annulation of 3-Aryl/Heteryl-2-(diazoacetyl)-1H-pyrroles: Synthesis of Benzo/Furo/Thieno[e]-Fused 1H-Indol-7-oles and Their Transformations. J. Org. Chem. 2019, 84, 10388-10401, DOI: 10.1021/acs.joc.9b01573;
- Galenko E. E., Novikov M. S., Shakirova F. M., Shakirova J. R., Kornyakov I. V., Bodunov V. A., Khlebnikov A. F. An Isoxazole Strategy for the Synthesis of 2,2’-Bipyridine Ligands: Symmetrical and Unsymmetrical 6,6’-Binicotinates, 2,2’-Bipyridine-5-carboxylates, and Their Metal Complexes. J. Org. Chem. 2019, 84, 3524-3536, DOI: 10.1021/acs.joc.9b00115;
- Shakirova J. R., Tomashenko O. A., Galenko E. E., Khlebnikov A. F., Hirva P., Starova G. L., Su S. H., Chou P. T., Tunik S. P.. Metalated Ir(III) Complexes Based on the Luminescent Diimine Ligands: Synthesis and Photophysical Study. Inorg. Chem. 2018, 57, 6853-6864, DOI: 10.1021/acs.inorgchem.8b00390;
- Galenko E. E.; Galenko A. V.; Novikov M. S.; Khlebnikov A. F.; Kudryavtsev I. V.; Terpilowski M. A.; Serebriakova M. K.; Trulioff A. S.; Goncharov N. V. 4-Diazo and 4-(Triaz-1-en-1-yl)-1H-pyrrole-2-carboxylates as Agents Inducing Apoptosis. ChemistrySelect 2017, 2, 7508-7513. doi: 10.1002/slct.201701538;
- Shakirova J. R., Tomashenko O. A., Grachova E. V., Starova G. L., Sizov V. V., Khlebnikov A. F., Tunik S. P. Gold(I)-alkynyl complexes with a new type N-donor heterocyclic ligand: Synthesis and photophysical properties. Eur. J. Inorg. Chem., 2017, 4180-4186DOI: 10.1002/ejic.201700731;
- Koshel E. I., Tomashenko O. A., Khlebnikov A. F., Gaginskaya E. R., Saifitdinova A. F., Tunik S. P. A new nuclear heterocyclic fluorescent dye – a promising tool for karyology and cytogenetics. Chromosome Res. 2016, 24 (Suppl 1), S29–S30. DOI 10.1007/s10577-016-9532-x;
- Konev A. S., Khlebnikov A. F., Levin O. V., Lukyanov D. A., Zorin I. M. Photocurrent in Multilayered Assemblies of Porphyrin–Fullerene Covalent Dyads: Evidence for Channels for Charge Transport. ChemSusChem. 2016, 9, 676–686. DOI:10.1002/cssc.201501686;
- Tomashenko O. A., Khlebnikov A. F., Mosiagin I. P., Novikov M. S., Grachova E. V., Shakirova J. R., Tunik S. P. A new heterocyclic skeleton with highly tunable absorbtion/emission wavelength via H-bonding RSC Adv. 2015, 5, 94551-94561; DOI: 10.1039/C5RA17755C.
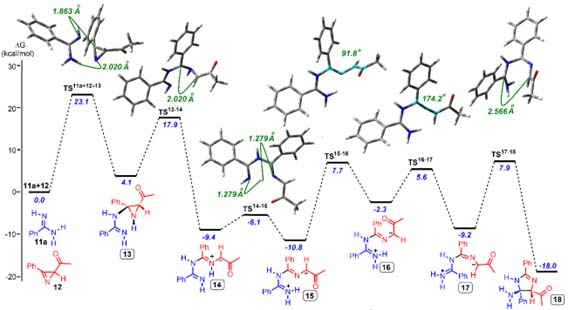
Квантово-химические расчеты энергетических характеристик молекул, реакций и равновесий.
- Galenko, E. E.; Novikov, M. S.; Bunev, A. S.; Khlebnikov, A. F. Acridine–Isoxazole and Acridine–Azirine Hybrids: Synthesis, Photochemical Transformations in the UV/Visible Radiation Boundary Region, and Anticancer Activity. Molecules 2024, 29, 1538. DOI: 10.3390/molecules29071538.
- Taishev, A. E.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Simple Access to Isoxazole-Containing Heterocyclic Hybrids: Isoxazole/Oxazole and Isoxazole/Pyridine. Russ. J. Gen. Chem. 2023, 93, 1246–1260, DOI: 10.1134/S1070363223050250.
- Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. [2 + 2] Cycloaddition/Retro-Electrocyclization/Decarboxylation Reaction Sequence: Access to 4‑Aminopyridines from Methylideneisoxazolones and Ynamines. J. Org. Chem. 2023, 88, 8854–8864. DOI: 10.1021/acs.joc.3c00654.
- Zanakhov, T. O.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Diazo Strategy for Intramolecular Azirine Ring Expansion: Rh(II)-Catalyzed Synthesis of 2‑Hydroxy-3-oxo-2,3-dihydro‑1H‑pyrrole-2-carboxylates. J. Org. Chem. 2022, 87, 15598–15607. DOI: 10.1021/acs.joc.2c02177.
- Kaminskiy, N. A.; Galenko, E. E.; Kryukova, M. A.; Novikov, M. S.; Khlebnikov, A. F. Reaction of α‑Diazopyrroles with Enamines: Synthesis of Pyrrolo[2,1‑c][1,2,4]triazines and α‑(1,2,5-Triazapenta-1,3-dienyl)pyrroles. J. Org. Chem. 2022, 87, 10485−10492, DOI: 10.1021/acs.joc.2c01102.
- Krivolapova, Yu. V.; Tomashenko, O. A.; Funt, L. D.; Spiridonova, D. V.; Novikov, M. S.; Khlebnikov, A. F. Azirine-triazole hybrids: selective synthesis of 5-(2H-azirin-2-yl)-, 5-(1H-pyrrol-2-yl)-1H-1,2,3-triazoles and 2-(5-(2H-azirin-2-yl)-1H-1,2,3-triazol-1-yl)pyridines. Org. Biomol. Chem. 2022, 20, 5434–5443, DOI: 10.1039/D2OB00908K.
- Efimenko N. I., Tomashenko O. A., Spiridonova D. V., Novikov M. S., Khlebnikov A. F. Nucleophile-Induced Rearrangement of 2H-Azirine-2-carbonyl Azides to 2-(1H-Tetrazol-1-yl)acetic Acid Derivatives. Org. Lett. 2021, 23, 6362–6366, DOI: 10.1021/acs.orglett.1c02157.
- Danilkina N. A., Govdi A. I., Khlebnikov A. F., Tikhomirov A. O., Sharoyko V. V., Ryazantsev M. N., ShtyrovA. A., Bräse S., Balova I. A. Heterocycloalkynes Fused to a Heterocyclic Core: Searching for an Island with Optimal Stability-Reactivity Balance. J. Am. Chem. Soc. 2021, 143, 16519–16537, DOI: 10.1021/jacs.1c06041.
- Serebryannikova, A. V.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Product selectivity of thermal Buchner reaction of methyl 2-(3-arylisoxazol-5-yl)-2-diazoacetates with benzene, naphthalene and mesitylene, and ring-opening/closing reaction of products. Tetrahedron 2021, 77, 132153, DOI: 10.1016/j.tet.2021.132153.
- Galenko E. E., Bodunov V. A., Kryukova M. A., Novikov M. S., Khlebnikov A. F. Buchner Reaction/Azirine Modification Approach Toward Cycloheptatriene Containing Nitrogen Heterocyclic Scaffolds. J. Org. Chem. 2021, 86, 4098–4111, DOI: 10.1021/acs.joc.0c02928.


