Календарь событий
Лекция профессора Shuji Akai «Lipase-metal combo catalyzed dynamic kinetic resolution» |
|||||
|
|
|
||||
|
|||||
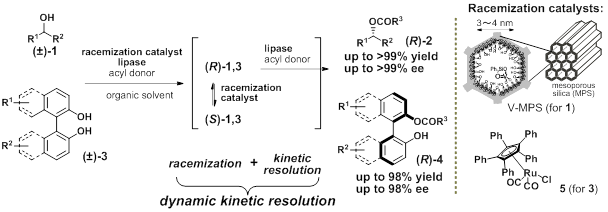
Over the past few decades, lipase-catalyzed dynamic kinetic resolution (DKR) of racemic secondary alcohols 1 has emerged as a practical method for the synthesis of enantiomerically pure compounds 2 in quantitative yields. DKR proceeds through an enzymatic kinetic resolution of racemic 1 along with the in situ racemization of the less reactive enantiomers in one pot.1 In this lecture, I present our original methods for DKR of (±)-1 to produce optically pure compounds 2. We have also invented lipase-catalyzed DKR of axially-chiral 2,2’-dihydroxy-1,1’-biaryls (±)-3, which has never been achieved so far. Our DKR protocol is based on a combined use of commercially available immobilized lipases and a specially designed racemization catalyst, V-MPS, in which oxovanadium species are covalently bound to the inner surface of mesoporous silica (MPS) with a pore size of 3–4 nm. Its small pores completely separate the lipases and oxovanadium moiety to produce perfect compatibility of these two catalysts in a single flask. Therefore, the racemates 1 were converted into the optically active esters (R)-2 in up to 99% isolated yield with 99% ee. Its practical utilities were demonstrated by the asymmetric syntheses of some bioactive natural products.2 On the other hand, a novel DKR of axially-chiral (±)-3 was born by our discovery that a ruthenium complex 5 facilitated the racemization of the configurationally stable optically active 3 at 35 °C. The detailed studies have clarified that the racemization proceeds through a SET process. By the combination of commercial lipase and the complex 5, DKR of binaphthols and biphenols (±)-3 bearing various substituents has been performed to produce the corresponding esters (R)-4 in up to 98% isolated yield and up to 98% ee.3 Well-investigated enantioselective oxidative biaryl couplings sometimes suffer from decreases in optical purity of products through radical mediated racemization. In contrast, such undesired racemizations are advantageous in our DKR. Another key feature of our method is sufficient stability of the acylated products against racemization under the DKR conditions.
1 Review: S. Akai, Chem. Lett., 2014, 43, 746. 2 S. Akai et al., Angew. Chem. Int. Ed. 2013, 52, 3654; Catal. Sci. Technol. 2016, 6, 5023; Green Chem. 2017, 19, 411; Bioorg. Med. Chem., 2018, 26, 1378. 3 S. Akai et al., Angew. Chem. Int. Ed. 2018, 57, 10278. |
|||||
| Место : аудитория 315 | |||||



 Состоится лекция профессора Shuji Akai (Osaka University, Japan) «Lipase-metal combo catalyzed dynamic kinetic resolution».
Состоится лекция профессора Shuji Akai (Osaka University, Japan) «Lipase-metal combo catalyzed dynamic kinetic resolution».